High Resolution 3D Printed ECM Scaffolds
01/13/20
Publication Title:
Development of High-Resolution Three-Dimensional-Printed Extracellular Matrix Scaffolds and Their Compatibility with Pluripotent Stem Cells and Early Retinal Cells
Advanced BioMatrix Products Used:
PhotoCol® Methacrylated Collagen, PhotoGel® Methacrylated Gelatin, PhotoHA® Methacrylated Hyaluronic Acid
How the products were used:
Our Methacrylated ECM's were used as a photocrosslinkable bioink for two-photon polymerization.
Article Abstract:
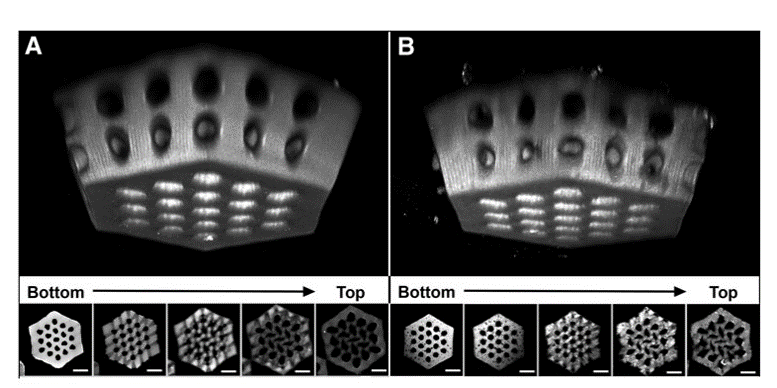
Purpose: Widely used approaches for retinal disease modeling and in vitro therapeutic testing can be augmented by using tissue-engineered scaffolds with a precise 3-dimensional structure. However, the materials currently used for these scaffolds are poorly matched to the biochemical and mechanical properties of the in vivo retina. Here, we create biopolymer-based scaffolds with a structure that is amenable to retinal tissue engineering and modeling.
Methods: Optimal two-photon polymerization (TPP) settings, including laser power and scanning speed, are identified for 4 methacrylated biopolymer formulations: collagen, gelatin, hyaluronic acid (HA), and a 50/50 mixture of gelatin/HA, each with methylene blue as a photoinitiator. For select formulations, fabrication accuracy and swelling are determined and biocompatibility is evaluated by using human induced pluripotent stem cells and rat postnatal retinal cells.
Results: TPP is feasible for each biopolymer formulation, but it is the most reliable for mixtures containing gelatin and the least reliable for HA alone. The mean size of microscaffold pores is within several microns of the intended value but the overall structure size is several times greater than the modeled volume. The addition of HA to gelatin scaffolds increases cell viability and promotes neuronal phenotype, including Tuj-1 expression and characteristic morphology.
Conclusion: We successfully determined a useful range of TPP settings for 4 methacrylated biopolymer formulations. When crosslinked, these extracellular matrix-derived molecules support the growth and attachment of retinal cells. We anticipate that when combined with existing patient-specific approaches, this technique will enable more efficient and accurate retinal disease modeling and therapeutic testing in vitro than current techniques allow.



