-
Collagen
-
Type I - Atelocollagen
- PureCol® Solution, 3 mg/ml (bovine) #5005
- Nutragen® Solution, 6 mg/ml (bovine) #5010
- FibriCol® Solution, 10 mg/ml (bovine) #5133
- PureCol® EZ Gel, Solution, 5 mg/ml (bovine) #5074
- PureCol® Lyophilized, 15 mg (bovine) #5006
- VitroCol® Solution, 3 mg/ml (human) #5007
- VitroCol® Lyophilized, 15 mg (human) #5008
-
Type I - Telocollagen
- TeloCol®-3 Solution, 3 mg/ml (bovine) #5026
- TeloCol®-6 Solution, 6 mg/ml (bovine) #5225
- TeloCol®-10 Solution, 10 mg/ml (bovine) #5226
- RatCol™ for 2D and 3D, Solution, 4 mg/ml (rat) #5153
- RatCol™ High Concentration, Solution, 10 mg/ml (rat)
- RatCol™ lyophilized, 100 mg (rat)
- RatCol™ for Coatings, Solution, 4 mg/ml (rat) #5056
- Type I - Insoluble Collagen
- Type I - Bioinks
- Type II Collagen
- Type III Collagen
- Type IV Collagen
- Collagen Standard
-
PureCol® Collagen Coated Plates
- Collagen Coated T-25 Flasks #5029
- Collagen Coated 6-well Plates #5073
- Collagen Coated 12-well Plates #5439
- Collagen Coated 24-well Plates #5440
- Collagen Coated 48-well Plates #5181
- Collagen Coated 96-well Plates #5072
- Collagen Coated 384-well Plates #5380-5EA
- Collagen Coated 100 x 20 mm Dishes #5028
- MatTek Glass-Bottom Dishes
- MatTek Multi-Well Plates
- Collagen Scaffolds
- Collagen Hybridizing Peptides
-
Type I - Atelocollagen
- Tunable Stiffness
- CytoSoft™ Rigidity Plates
-
Bioprinting
- Support Slurry for FRESH Bioprinting
-
Bioinks for Extrusion Bioprinting
- Lifeink® 200 Collagen Bioink (35 mg/ml) #5278
- Lifeink® 220 Collagen Bioink (70 mg/ml) #5343
- Lifeink® 240 Acidic Collagen Bioink (35 mg/ml) #5267
- Lifeink® 260 Acidic Collagen Bioink (70 mg/ml) #5358
- GelMA Bioink
- GelMA A Bioink
- GelMA C Bioink
- Pluronic F-127 40% Sterile Solution
- GelMA 20% Sterile Solution
- Alginate 5% Sterile Solution
- Photoinitiators
- Bioinks for BIONOVA X
- Bioinks for Lumen X
- DLP Printing Consumables
-
Create Your Own Bioinks
- PhotoCol® Methacrylated Collagen
- PhotoGel® Methacrylated Gelatin 95% DS
- PhotoGel® Methacrylated Gelatin 50% DS
- PhotoHA®-Stiff Methacrylated Hyaluronic Acid
- PhotoHA®-Soft Methacrylated Hyaluronic Acid
- PhotoAlginate® Methacrylated Alginate
- PhotoDextran® Methacrylated Dextran
- PEGDA (Various Molecular Weights)
- Silk Fibroin, Solution
- PhotoSericin® Methacrylated Sericin
- Bioprinters
-
3D Hydrogels
- Thermoreversible Hydrogel
- Silk Fibroin
-
Type I Collagen for 3D Hydrogels
- PureCol® Solution, 3 mg/ml (bovine) #5005
- Nutragen® Solution, 6 mg/ml (bovine) #5010
- FibriCol® Solution, 10 mg/ml (bovine) #5133
- PureCol® EZ Gel, Solution, 5 mg/ml (bovine) #5074
- VitroCol® Solution, 3 mg/ml (human) #5007
- TeloCol®-3 Solution, 3 mg/ml (bovine) #5026
- TeloCol®-6 Solution, 6 mg/ml (bovine) #5225
- TeloCol®-10 Solution, 10 mg/ml (bovine) #5226
- RatCol® for 3D gels, Solution, 4 mg/ml (rat) #5153
- HyStem® Thiolated Hyaluronic Acid
- Methacrylated Collagen
- Methacrylated Gelatin
- Methacrylated Hyaluronic Acid
- Diacrylates
- Collagen Sponges
- Methacrylated Polysaccharides
- Spheroids and Organoids
- Extracellular Matrices
- HyStem / Hyaluronic Acid
-
Adhesion Peptides / Proteins
-
Recombinant Adhesion Proteins
- CD2, 0.5 mg/ml #5086
- CDH3, 0.5 mg/ml #5124
- CDH13, 0.5 mg/ml #5125
- CD14, 0.5 mg/ml #5089
- CDH18, 0.5 mg/ml #5090
- CD40, 0.5 mg/ml #5093
- CD86, 0.5 mg/ml #5096
- CD164, 0.5 mg/ml #5100
- CD270, 0.5 mg/ml #5127
- CD274, 0.5 mg/ml #5126
- CD276, 0.5 mg/ml #5123
- E-Cadherin (CD324), 0.5 mg/ml #5085
- ICAM2, 0.5 mg/ml #5107
- Adhesion Peptides
- Collagen Hybridizing Peptides
-
Recombinant Adhesion Proteins
- Reagents
- Assays
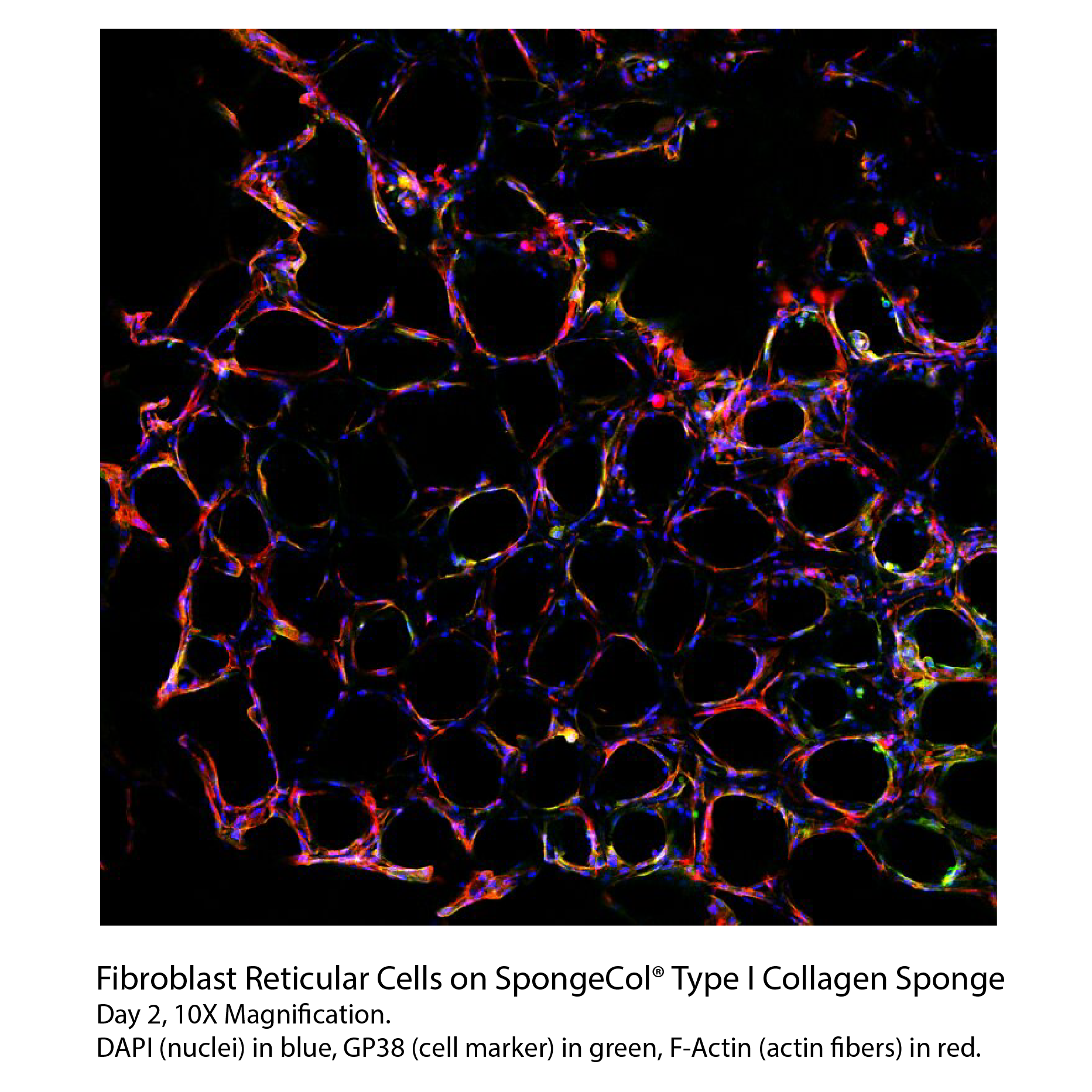
SpongeCol®
Collagen Sponges with Columnar Pore Architecture
Catalog #5135

SpongeCol®
Collagen Sponges with Columnar Pore Architecture
Catalog #5135
SpongeCol® is a type I collagen sponge with an interpenetrating, columnar pore architecture. SpongeCol® provides an increased surface area for cell attachment, growth and migration for tissue engineering applications.
Product Description
SpongeCol® is a collagen sponge with an interpenetrating, columnar porous architecture structure. SpongeCol® contains unique columnar porous network which permits cells and nutrients to flow completely through the interpenetrating pores and provides an increased surface area for cell attachment, growth and migration.
SpongeCol® is composed of highly purified Type I collagen (bovine) which best supports the attachment, proliferation, and function of cells. The collagen is lightly cross-linked for increased mechanical strength and durability for short and long term tissue culture yet is still biodegradable over the longer-term. The diameter of the pores ranges from 100 to 400 micron with the average diameter being approximately 200 microns. The collagen disc is approximately 4 mm or 21 mm in diameter and 1.5 mm thick. The sponge discs fits into a 96-well (4 mm disc) or 12-well (21 mm disc) culture plate or sanitary luer connectors for flow perfusion. Each package contains 25 (4 mm) or 5 (21 mm) collagen sponge discs.
This product is terminally sterilized and ready-to-use.
| Parameter, Testing, and Method | SpongeCol® #5135 |
| Disc Diameter | 4mm or 21mm |
| Package Size | 25 or 5 Sponges/Pack |
| Disc Thickness | 1.5 mm |
| Pore Size | ~200 micron diameter with a range of 100-400 micron |
| Endotoxin - LAL | < 1.0 EU/mL |
| Storage Temperature | Room Temperature |
| Shelf Life | Minimum of 6 months from date of receipt |
| Sterilization Method | Irradiation |
| Sterility - USP modified | No growth |
| Collagen Source | Purified Type I Collagen |
| Collagen Purity - Silver Staining | >99% |
Directions for Use
Download the full PDF for SpongeCol® 4mm discs or SpongeCol® 21mm discs, or continue reading below:
A. Preparation and Seeding:
Note: Cell attachment to the sponge is generally the most critical step in tissue culture. Temperature, pH, gas exchange and cell concentration can affect the rate and efficiency of attachment. Optimum seeding rate depends on the type of cell being cultured.
- Aseptically remove the sponge discs from the packaging in a laminar flow work station.
- Carefully place the sponges into the wells of a 12 well (21mm sponge) or 96 well (4mm sponge) tissue culture plate using a sterile instrument. Be careful not to damage the sponge as it is being transferred. It is recommended to use non-treated tissue culture plasticware.
Note: Tissue-coated plasticware may need to be coated with agarose to prevent cell attachment to the plastic instead of the sponge. - Suspend cells at desired concentration (1×104 – 1×105 cells/mL) and dispense sufficient volume of cell solution on top of the sponge placed in the well. Note: An alternative method is to suspend cells in a neutralized collagen solution (such as PureCol® type I collagen Catalog #5005-100ML or PureCol® EZ Gel Catalog #5074-35ML). Dispense collagen/cell solution on top of the sponge placed in the well.
- Transfer to a 37ºC incubator for about 1 – 2 hours to allow for initial cell attachment.
- After 1 – 2 hour, remove the plate from the incubator and check for cell attachment. Additional testing may be required to optimize the time it takes for the cells to attach to the sponge. Check the morphology of the cells. Cell adherence and spreading will dictate the time for attachment.
- Once the cells have adequately attached to the sponge, increase the final volume in each well to fully cover and provide adequate medium for the culture system.
B. Changing the Media:
- Change the media 12 to 24 hours after the initial seeding. The frequency of changes will be determined by cell type, cell attachment efficiency, pH (maintain at pH 7.0 to 7.4) utilization of medium nutrients available to cultures. More frequent medium changes may be required compared to 2D culture systems.
C. Harvesting of Cells:
Note: Protease digestion is the standard method of releasing cells from the sponges. The strength of the attachment of the cells to the collagen sponges will vary from cell line to cell line. The enzyme concentration and digestion time will vary depending upon the activity of the enzyme and the confluence of the cells. Collagenase and/or trypsin may be the preferred method.
- Washing the sponge with EDTA-PBS may assist the protease digestion. Add sufficient volume to cover the sponge.
- Aspirate the EDTA-PBS solution from the well.
- Add sufficient dissociation solution to the well to fully over the sponge.
- Transfer to a 37ºC incubator. Check for cell detachment periodically for cell detachment.
- Once the cells have fully detached, remove the cells and dispense in a centrifuge tube.
- Centrifuge the cells as require.
Product Cell Assay
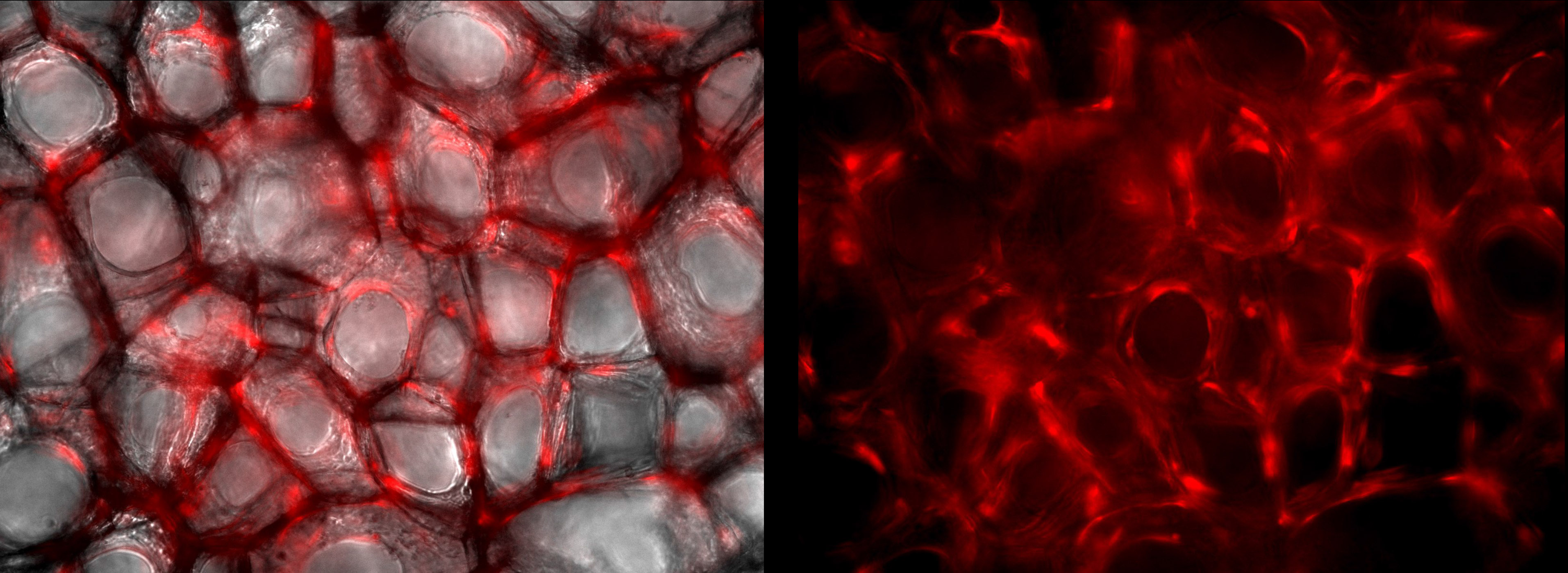
Endothelial Fibroblasts at 100X magnification. Images taken after 8 days of cell culture within SpongeCol®. Cells stained with Endothelial RSP.
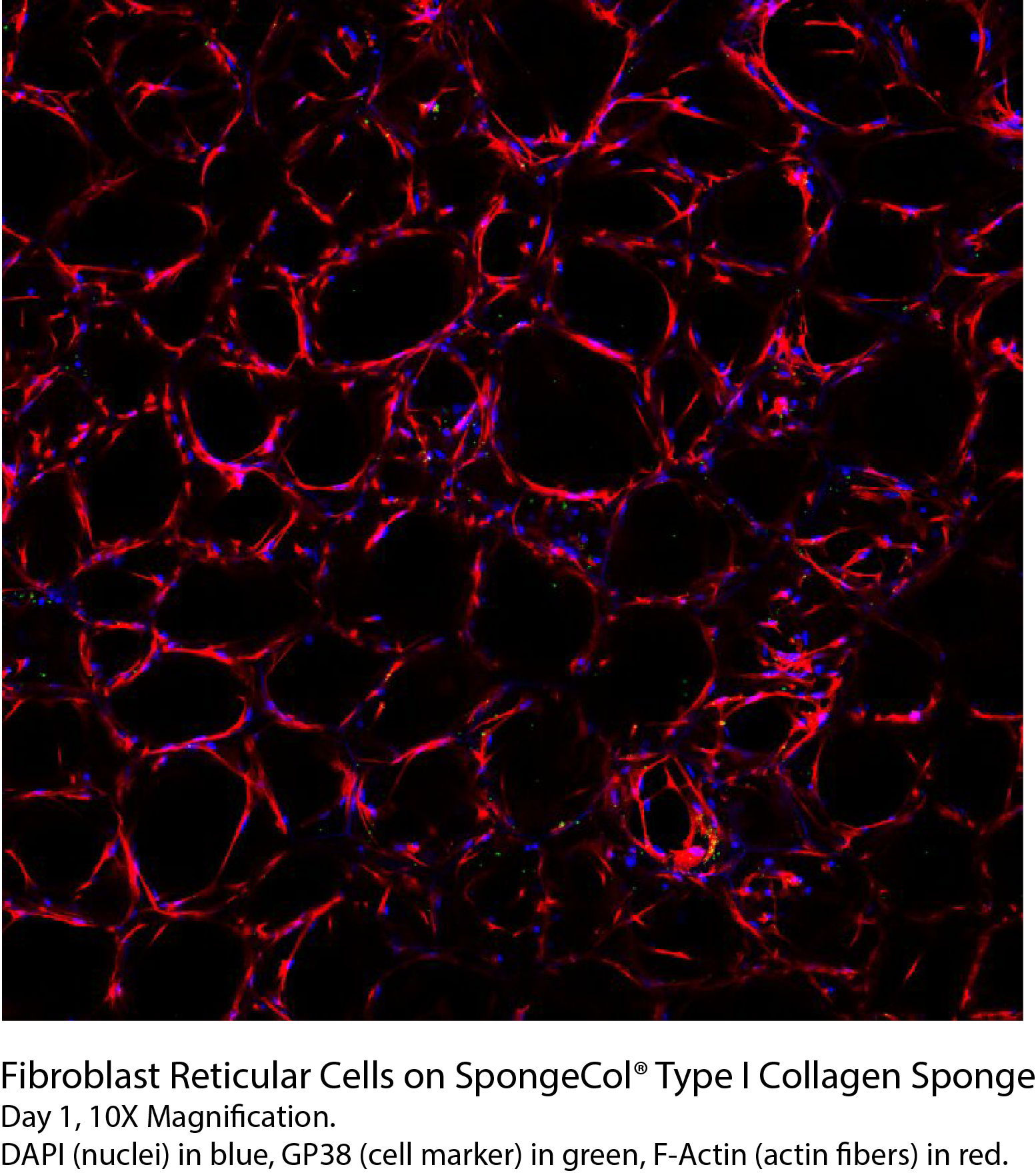
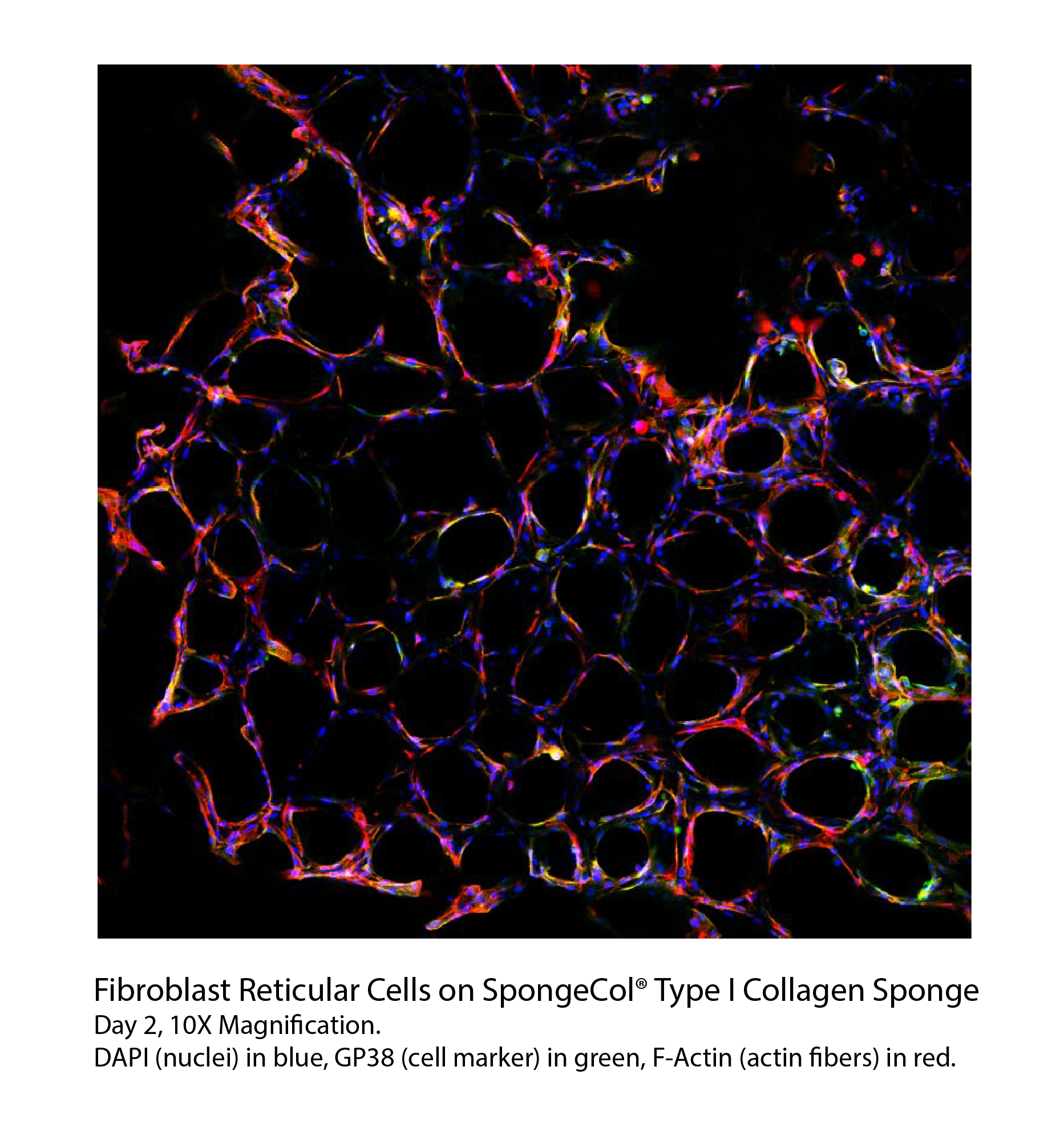

SpongeCol® hydrated in 1X PBS, 5x magnification
Product References
References for SpongeCol®:
1. Mak, W. C., et al. “Thermo-Rheological Responsive Microcapsules for Time-Dependent Controlled Release of Human Mesenchymal Stromal Cells.” Biomater. Sci., vol. 5, no. 11, 2017, pp. 2241–2250., doi:10.1039/c7bm00663b.
2. Udhayakumar, Sivalingam, et al. “l-Arginine Intercedes Bio-Crosslinking of a Collagen–Chitosan 3D-Hybrid Scaffold for Tissue Engineering and Regeneration: in Silico, in Vitro, and in Vivo Studies.” RSC Advances, vol. 7, no. 40, 2017, pp. 25070–25088., doi:10.1039/c7ra02842c.
3. Ferreira, L.p., et al. “Design of Spherically Structured 3D in Vitro Tumor Models -Advances and Prospects.” Acta Biomaterialia, 2018, doi:10.1016/j.actbio.2018.05.034.
4. Hatsell, Sarah J., et al. “ACVR1 R206H Receptor Mutation Causes Fibrodysplasia Ossificans Progressiva by Imparting Responsiveness to Activin A.” Science Translational Medicine, vol. 7, no. 303, Feb. 2015, doi:10.1126/scitranslmed.aac4358.
5. Anton-Sales, I., Beekmann, U., Laromaine, A., Roig, A. & Kralisch, D. Opportunities of Bacterial Cellulose to Treat Epithelial Tissues. Current Drug Targets20,808–822 (2019).
6. Nosenko, M. A. et al. Fibroblasts upregulate expression of adhesion molecules and promote lymphocyte retention in 3D fibroin/gelatin scaffolds. Bioactive Materials 6, 3449–3460 (2021).
Product Certificate of Analysis
No result for .
Product Videos
Safety and Documentation
Product Disclaimer
This product is for R&D use only and is not intended for human or other uses. Please consult the Material Safety Data Sheet for information regarding hazards and safe handling practices.