-
Collagen
-
Type I - Atelocollagen
- PureCol® Solution, 3 mg/ml (bovine) #5005
- Nutragen® Solution, 6 mg/ml (bovine) #5010
- FibriCol® Solution, 10 mg/ml (bovine) #5133
- PureCol® EZ Gel, Solution, 5 mg/ml (bovine) #5074
- PureCol® Lyophilized, 15 mg (bovine) #5006
- VitroCol® Solution, 3 mg/ml (human) #5007
- VitroCol® Lyophilized, 15 mg (human) #5008
-
Type I - Telocollagen
- TeloCol®-3 Solution, 3 mg/ml (bovine) #5026
- TeloCol®-6 Solution, 6 mg/ml (bovine) #5225
- TeloCol®-10 Solution, 10 mg/ml (bovine) #5226
- RatCol™ for 2D and 3D, Solution, 4 mg/ml (rat) #5153
- RatCol™ High Concentration, Solution, 10 mg/ml (rat)
- RatCol™ lyophilized, 100 mg (rat)
- RatCol™ for Coatings, Solution, 4 mg/ml (rat) #5056
- Type I - Insoluble Collagen
- Type I - Bioinks
- Type II Collagen
- Type III Collagen
- Type IV Collagen
- Collagen Standard
-
PureCol® Collagen Coated Plates
- Collagen Coated T-25 Flasks #5029
- Collagen Coated 6-well Plates #5073
- Collagen Coated 12-well Plates #5439
- Collagen Coated 24-well Plates #5440
- Collagen Coated 48-well Plates #5181
- Collagen Coated 96-well Plates #5072
- Collagen Coated 384-well Plates #5380-5EA
- Collagen Coated 100 x 20 mm Dishes #5028
- MatTek Glass-Bottom Dishes
- MatTek Multi-Well Plates
- Collagen Scaffolds
- Collagen Hybridizing Peptides
-
Type I - Atelocollagen
- Tunable Stiffness
- CytoSoft™ Rigidity Plates
-
Bioprinting
- Support Slurry for FRESH Bioprinting
-
Bioinks for Extrusion Bioprinting
- Lifeink® 200 Collagen Bioink (35 mg/ml) #5278
- Lifeink® 220 Collagen Bioink (70 mg/ml) #5343
- Lifeink® 240 Acidic Collagen Bioink (35 mg/ml) #5267
- Lifeink® 260 Acidic Collagen Bioink (70 mg/ml) #5358
- GelMA Bioink
- GelMA A Bioink
- GelMA C Bioink
- Pluronic F-127 40% Sterile Solution
- GelMA 20% Sterile Solution
- Alginate 5% Sterile Solution
- Photoinitiators
- Bioinks for BIONOVA X
- Bioinks for Lumen X
- DLP Printing Consumables
-
Create Your Own Bioinks
- PhotoCol® Methacrylated Collagen
- PhotoGel® Methacrylated Gelatin 95% DS
- PhotoGel® Methacrylated Gelatin 50% DS
- PhotoHA®-Stiff Methacrylated Hyaluronic Acid
- PhotoHA®-Soft Methacrylated Hyaluronic Acid
- PhotoAlginate® Methacrylated Alginate
- PhotoDextran® Methacrylated Dextran
- PEGDA (Various Molecular Weights)
- Silk Fibroin, Solution
- PhotoSericin® Methacrylated Sericin
- Bioprinters
-
3D Hydrogels
- Thermoreversible Hydrogel
- Silk Fibroin
-
Type I Collagen for 3D Hydrogels
- PureCol® Solution, 3 mg/ml (bovine) #5005
- Nutragen® Solution, 6 mg/ml (bovine) #5010
- FibriCol® Solution, 10 mg/ml (bovine) #5133
- PureCol® EZ Gel, Solution, 5 mg/ml (bovine) #5074
- VitroCol® Solution, 3 mg/ml (human) #5007
- TeloCol®-3 Solution, 3 mg/ml (bovine) #5026
- TeloCol®-6 Solution, 6 mg/ml (bovine) #5225
- TeloCol®-10 Solution, 10 mg/ml (bovine) #5226
- RatCol® for 3D gels, Solution, 4 mg/ml (rat) #5153
- HyStem® Thiolated Hyaluronic Acid
- Methacrylated Collagen
- Methacrylated Gelatin
- Methacrylated Hyaluronic Acid
- Diacrylates
- Collagen Sponges
- Methacrylated Polysaccharides
- Spheroids and Organoids
- Extracellular Matrices
- HyStem / Hyaluronic Acid
-
Adhesion Peptides / Proteins
-
Recombinant Adhesion Proteins
- CD2, 0.5 mg/ml #5086
- CDH3, 0.5 mg/ml #5124
- CDH13, 0.5 mg/ml #5125
- CD14, 0.5 mg/ml #5089
- CDH18, 0.5 mg/ml #5090
- CD40, 0.5 mg/ml #5093
- CD86, 0.5 mg/ml #5096
- CD164, 0.5 mg/ml #5100
- CD270, 0.5 mg/ml #5127
- CD274, 0.5 mg/ml #5126
- CD276, 0.5 mg/ml #5123
- E-Cadherin (CD324), 0.5 mg/ml #5085
- ICAM2, 0.5 mg/ml #5107
- Adhesion Peptides
- Collagen Hybridizing Peptides
-
Recombinant Adhesion Proteins
- Reagents
- Assays
Insoluble Collagen
Type I Collagen, Insoluble Fibrous Powder (Bovine)
Catalog #5162
Insoluble Collagen
Type I Collagen, Insoluble Fibrous Powder (Bovine)
Catalog #5162
Type I bovine collagen, lyophilized fibrous powder is extracted from bovine flexor tendon. The collagen is polymeric insoluble collagen. This product can be readily prepared into such forms as tissue scaffolds, foams, sponges, suspensions, coatings, putties, films and sheets but does not form hydrogels.
Product Description
Type I bovine collagen, lyophilized fibrous powder, Catalog Number 5162, is extracted from bovine flexor tendon with the raw material sourced from closed/controlled herds of animals. Because the collagen is extracted from tendon, the material is more naturally crosslinked. The collagen is polymeric insoluble collagen. The manufacturing processes comply with stringent quality standards that have proven to yield a high quality product with lot-to-lot consistency. This product has a purity of >96% with Type II and Type III collagens not detectable.
This product is supplied as a lyophilized fibrous powder in a 1 gram package size. Bioburden and endotoxin levels are tested – this product is not considered sterile.
This collagen product is naturally cross-linked yielding a robust material for applications which require structure and strength. This product can be readily prepared into such forms as tissue scaffolds, foams, sponges, suspensions, coatings, putties, films and sheets but does not form hydrogels. Using typical cross-linking methods, this material can be tuned for optimal in vivo resorption. This collagen product is ideal for tissue engineering applications and uses with inorganic and biomaterials.
The product is provided in user-friendly packaging for use and storage. Avoid extended open-air exposure to environment since this material is hygroscopic.
| Parameter, Testing, and Method | Insoluble Type I Collagen Fibrous Powder #5162 |
| Form | Lyophilized Fibrous Powder |
| Package Size | 1 gram |
| Storage Temperature | Room temperature |
| Shelf Life | Minimum of 6 months from date of receipt |
|
Collagen Purity |
>96% |
| Endotoxin (LAL) | <20.0 EU/mL |
| Bioburden | <200 cfu/gram |
| Source | Bovine Flexor Tendon |
| Amino Acid Analysis | Characteristic |
Directions for Use
Download the full PDF version or continue reading below:
Note: The following procedure is based on preparation of 1 gram of collagen in 100 ml to initially prepare a 10 mg/ml collagen suspension. Smaller quantities and volumes may be used but the same ratios of collagen and solutions should be used.
- Weigh out 1 gram of collagen fibrous powder.
- Reconstitute 1 gram of collagen with 50 ml of cold purified water (50% of the final volume).
- Stir the collagen with the water continuously mixing for a minimum of 15 minutes until the collagen is fully wetted and the suspension appears to be a semi-solution.
- Add 50 ml of cold 0.02 M HCl to the collagen mixture and stir for a minimum of 10 minutes. This will yield a collagen concentration of 10 mg/ml with the suspension continuing to appear as a semi-solution.
- Measure the pH – the mixture should have a pH of 2 to 3.
- Using Waring stick blender or equivalent, homogenize the collagen mixture for a minimum of 15 minute at a high speed ensuring that the collagen is fully homogenized. Ensure that the temperature of mixture does not exceed 24°C. Upon completion, there should be very few visual solids in the viscous suspension. Air bubbles with be prevalent.
- To remove air bubbles, stir the solution on a stir plate and pull a vacuum on the suspension. This will remove the air bubbles.
- At this point if a collagen concentration of less than 10 mg/ml is desired, the collagen can be diluted with 0.01 M HCl.
Product Q & A
Please review our collagen hydrogel whitepapers found here: https://advancedbiomatrix.com/3d-collagen-hydrogel-stiffness.html
Please visit our collagen gelation diagnosis page: https://advancedbiomatrix.com/collagen-gelation-diagnosis.html
Please view our white paper here:
https://advancedbiomatrix.com/recommended-volumes-for-coatings-and-hydrogels.html
We completed a study to show that DNA is completely destroyed at pH 2, and demonstrated that our collagen products do not contain DNA.
The collagen is fully hydrolyzed. The amino acid analysis is done using the Waters AccQ-Tag derivatization method. During the acid hydrolysis step, asparagine (N) is converted to aspartic acid (D) and glutamine (Q) is converted to glutamic acid (E). Tryptophan (W), if present, is destroyed during acid hydrolysis. Experimentally, one can determine the picomoles (pmol) of each amino acid per injected detected using amino acid standards.For the concentration determination, the total number of pmol of each amino acid is summed to get the total pmol of the 18 amino acids detected. The total pmol amino acids is divided by the theoretical number of amino acid residues in collagen based on the published sequence. The result is the pmol of collagen injected. The result is then multiplied by the dilution and 300,000 is used as the collagen molecular weight to get to mg/mL. The molecular weight of collagen is not well agreed upon.
Diluting with 1X PBS (rather than water or 0.01 N HCl) would have an effect for coating purposes. It would change the pH of the diluted collagen solution from acid to neutral pH. The pH change will transform the collagen molecules from a molecular form to a fibrillar form; and then the nature of coating surface will be changed from a monomeric coating to a fibrillar coating.
We use the following antibodies from SouthernBiotech:
1. 1310-02 – Goat Anti-Type I Collagen-FITC
2. 1310-08 – Goat Anti-Type I Collagen-BIOT
The major collagen molecular species in our Type I collagen products are monomers (approx. 70%), but there are dimers, trimers and a few percentages of oligomers too (approx. 30%) with some minor amounts of collagen fragments. The collagen monomer is a rod shaped molecule with 300 nm in length and 1.5 nm in diameter. The dimer, trimer and oligomer are 600 nm, 900nm and even longer in length respectively. According to the coating procedures, the collagen molecules are attached to the charged polystyrene surface randomly by charge or affinity in acid conditions during the 1-2 hrs incubation period at 37°C, and any unattached materials are removed by aspiration and rinsing. Therefore, the coated surface is a single layer of collagen monomer, dimer, trimer and oligomer mixtures. The thickness of the mono-molecular layer is dependent on how those molecules are attached on the surface. The coating density thickness would generally be characterized as a 1 molecule thickness which could be ranging from a few nanometers to a few hundred nanometers with the whole surface being covered by collagen.
The net charge of Type I collagen products’ (PureCol®, Bovine Collagen and VitroCol®, Human Collagen) molecule is directly related to the pH. At an acidic pH, the amino acids (zwitterions) along the collagen molecule are positively charged, making the entire collagen molecule positive. At the isoelectric point (or zone) of collagen, around pH 7-8, the amino acids along the collagen molecule are positively and negatively charged, making the net charge of the collagen molecule close to zero. At a basic pH, the amino acids along the collagen molecule were negatively charged, making the entire collagen molecule negative.
Further, the nature of the charge of the collagen coating surface will be dependent on the type of coating applied. For a monomeric collagen coatings when the collagen is applied under an acidic pH condition, the surface is positively charged. If the surface is rinsed with pH neutral buffer or media then it will change the charge of the collagen surface net charge close to zero. For a 3D gel coating, the collagen prepared under neutral pH; the net charge of the collagen surface is close to zero.
Using rotary shadowing technique under transmission electron microscopy, it was found that our collagen, on average, consists of approximately 80% monomers, 13% dimers, trimers, and oligomers with the remaining 7% collagen fragments.
Yes. The collagen molecule in PureCol, Nutragen, VitroCol, and all of our other Atelo collagen products were prepared from native collagen matrix by pepsin treatment under controlled conditions to remove the non-helical portion, telo-peptides, only and the helical portion is intact. In this case, the enzymatic active sites for MMP (Matrix Metalloproteinase), such as for Mammalian Collagenase Matrix Metalloproteinase 8 (MMP-8), on the molecule was preserved.
These pepsin treated collagen products should behave as native intact collagen.
TGF beta would have been digested with the pepsin enzymatic digestion step. It was undetectable by SDS PAGE silver stain as well. We didn’t do any specific measurements by ELISA however but presences of TGF beta is not anticipated.
We primarily use the Biuret method, but we also use BCA, AAA, and hydroxyl-proline assays.
- Collagen solutions that are frozen tend to have issues forming 3D hydrogels, and will likely not work. The solutions should still be good for 2D coatings.
- Collagen solutions that are left out at room temperature for extended periods of time may show signs of degradation, which will affect the formation of 3D hydrogels. It is likely still fine for 2D coatings.
Our recommendation is this: If you are using the product directly for a publication, we highly suggest buying a new bottle if the one you have was compromised.
In the publication "Fibroblast Promotes Head and Neck Squamous Cell Carcinoma Cells Invasion through Mechanical Barriers in 3D Collagen Microenvironments," they reported the porosity of FibriCol® type I atelocollagen hydrogels prepared at various concentrations, as reported below:
1 mg/ml = 5.7 + 2.3 μm
2 mg/ml = 3.7 + 1.5 μm
4 mg/ml = 1.3 + 0.7 μm
8 mg/ml = 0.8 + 0.4 μm
We can say that our coating saturates the available culture surface with a dense layer of collagen that is stable and cannot be washed off.
The “coating density” is represented by an array of molecules packaged at maximum, area-filling density, in a mono-molecular layer onto the surface.
The absolute mass of coated collagen per surface area depends on the Stoke’s radius and shape of the specific ECM being coated, yielding coating densities in the range of 0.2 – 0.4 micrograms of protein per square centimeter of available surface.
Our bovine collagen comes in two formats: Atelocollagen (enzyme extracted) and Telocollagen (acid extracted). The rat tail collagen we offer is Telocollagen.
When comparing both bovine and rat tail telocollagen products, some consider rat tail collagen to be more immunogenic compared to bovine.
For the triple helix section, rat tail collagen is 97.5% similar to human, while bovine is 99.3% similar.
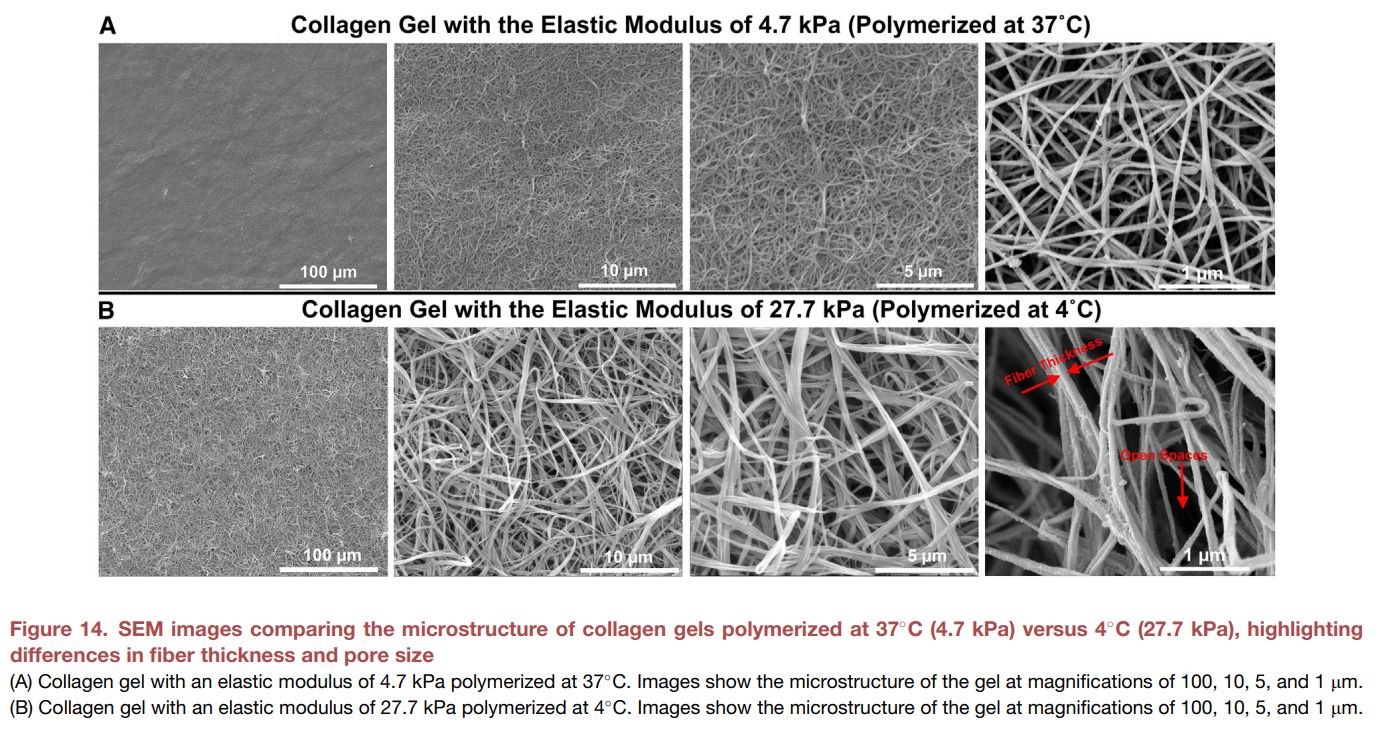
From the publication https://doi.org/10.1016/j.matt.2025.102094, we see that telocollagen hydrogels polymerized at 37C form thinner fibers, with decreased porosity, and softer hydrogels.
Polymerizing telocollagen at 4C forms thicker fibers, increased porosity and firmer hydrogels. The stiffness values were evaluated by individual fibers, not bulk hydrogel stiffness.
Product References
References for Insoluble Collagen:
Ahmed, Raju, Monjurul Haq, and Byung-Soo Chun. "Characterization of marine derived collagen extracted from the by-products of bigeye tuna (Thunnus obesus)." International journal of biological macromolecules (2019).
Divakar, Prajan, Kaiyang Yin, and Ulrike GK Wegst. "Values and property charts for anisotropic freeze-cast collagen scaffolds for tissue regeneration." Data in brief 22 (2019): 502-507.
Divakar, Prajan, Kaiyang Yin, and Ulrike GK Wegst. "Anisotropic freeze-cast collagen scaffolds for tissue regeneration: How processing conditions affect structure and properties in the dry and fully hydrated states." Journal of the mechanical behavior of biomedical materials 90 (2019): 350-364.
Product Certificate of Analysis
No result for .
Product Videos
Product Disclaimer
This product is for R&D use only and is not intended for human or other uses. Please consult the Material Safety Data Sheet for information regarding hazards and safe handling practices.