-
Collagen
-
Type I - Atelocollagen
- PureCol® Solution, 3 mg/ml (bovine) #5005
- Nutragen® Solution, 6 mg/ml (bovine) #5010
- FibriCol® Solution, 10 mg/ml (bovine) #5133
- PureCol® EZ Gel, Solution, 5 mg/ml (bovine) #5074
- PureCol® Lyophilized, 15 mg (bovine) #5006
- VitroCol® Solution, 3 mg/ml (human) #5007
- VitroCol® Lyophilized, 15 mg (human) #5008
-
Type I - Telocollagen
- TeloCol®-3 Solution, 3 mg/ml (bovine) #5026
- TeloCol®-6 Solution, 6 mg/ml (bovine) #5225
- TeloCol®-10 Solution, 10 mg/ml (bovine) #5226
- RatCol™ for 2D and 3D, Solution, 4 mg/ml (rat) #5153
- RatCol™ High Concentration, Solution, 10 mg/ml (rat)
- RatCol™ lyophilized, 100 mg (rat)
- RatCol™ for Coatings, Solution, 4 mg/ml (rat) #5056
- Type I - Insoluble Collagen
- Type I - Bioinks
- Type II Collagen
- Type III Collagen
- Type IV Collagen
- Collagen Standard
-
PureCol® Collagen Coated Plates
- Collagen Coated T-25 Flasks #5029
- Collagen Coated 6-well Plates #5073
- Collagen Coated 12-well Plates #5439
- Collagen Coated 24-well Plates #5440
- Collagen Coated 48-well Plates #5181
- Collagen Coated 96-well Plates #5072
- Collagen Coated 384-well Plates #5380-5EA
- Collagen Coated 100 x 20 mm Dishes #5028
- MatTek Glass-Bottom Dishes
- MatTek Multi-Well Plates
- Collagen Scaffolds
- Collagen Imaging Reagents
-
Type I - Atelocollagen
- Tunable Stiffness
- CytoSoft™ Rigidity Plates
-
Bioprinting
- Support Slurry for FRESH Bioprinting
-
Bioinks for Extrusion Bioprinting
- Lifeink® 200 Collagen Bioink (35 mg/ml) #5278
- Lifeink® 220 Collagen Bioink (70 mg/ml) #5343
- Lifeink® 240 Acidic Collagen Bioink (35 mg/ml) #5267
- Lifeink® 260 Acidic Collagen Bioink (70 mg/ml) #5358
- GelMA Bioink
- GelMA A Bioink
- GelMA C Bioink
- Pluronic F-127 40% Sterile Solution
- GelMA 20% Sterile Solution
- Alginate 5% Sterile Solution
- Photoinitiators
- Bioinks for BIONOVA X
- Bioinks for Lumen X
- DLP Printing Consumables
-
Create Your Own Bioinks
- PhotoCol® Methacrylated Collagen
- PhotoGel® Methacrylated Gelatin 95% DS
- PhotoGel® Methacrylated Gelatin 50% DS
- PhotoHA®-Stiff Methacrylated Hyaluronic Acid
- PhotoHA®-Soft Methacrylated Hyaluronic Acid
- PhotoAlginate® Methacrylated Alginate
- PhotoDextran® Methacrylated Dextran
- PEGDA (Various Molecular Weights)
- Silk Fibroin, Solution
- PhotoSericin® Methacrylated Sericin
- Bioprinters
-
3D Hydrogels
- Thermoreversible Hydrogel
- Silk Fibroin
-
Type I Collagen for 3D Hydrogels
- PureCol® Solution, 3 mg/ml (bovine) #5005
- Nutragen® Solution, 6 mg/ml (bovine) #5010
- FibriCol® Solution, 10 mg/ml (bovine) #5133
- PureCol® EZ Gel, Solution, 5 mg/ml (bovine) #5074
- VitroCol® Solution, 3 mg/ml (human) #5007
- TeloCol®-3 Solution, 3 mg/ml (bovine) #5026
- TeloCol®-6 Solution, 6 mg/ml (bovine) #5225
- TeloCol®-10 Solution, 10 mg/ml (bovine) #5226
- RatCol® for 3D gels, Solution, 4 mg/ml (rat) #5153
- HyStem® Thiolated Hyaluronic Acid
- Methacrylated Collagen
- Methacrylated Gelatin
- Methacrylated Hyaluronic Acid
- Diacrylates
- Collagen Sponges
- Methacrylated Polysaccharides
- Spheroids and Organoids
- Extracellular Matrices
- HyStem / Hyaluronic Acid
-
Adhesion Peptides / Proteins
-
Recombinant Adhesion Proteins
- CD2, 0.5 mg/ml #5086
- CDH3, 0.5 mg/ml #5124
- CDH13, 0.5 mg/ml #5125
- CD14, 0.5 mg/ml #5089
- CDH18, 0.5 mg/ml #5090
- CD40, 0.5 mg/ml #5093
- CD86, 0.5 mg/ml #5096
- CD164, 0.5 mg/ml #5100
- CD270, 0.5 mg/ml #5127
- CD274, 0.5 mg/ml #5126
- CD276, 0.5 mg/ml #5123
- E-Cadherin (CD324), 0.5 mg/ml #5085
- ICAM2, 0.5 mg/ml #5107
- Adhesion Peptides
- Collagen Hybridizing Peptides
-
Recombinant Adhesion Proteins
- Reagents
- Assays
Gelin-S Version 2.0
Thiol-Modified Gelatin
Catalog #GS230F #GS231F
Gelin-S Version 2.0
Thiol-Modified Gelatin
Catalog #GS230F #GS231F
Gelin-S is a thiol-modified gelatin that comes as a lyophilized powder. Gelin-S is a component of the HyStem®-C and HyStem®-HP kits, but can be purchased separately here. Comes with 10 mL of Buffer B.
Product Description
VERSION 2.0 INFORMATION:
HyStem Version 1.0 kit components were reconstituted in degassed water resulting in a pH neutral isotonic salt hydrogel. To create a more consistent and advanced product, we have made the following change:
HyStem V2.0 kit components will now be reconstituted with a buffered 1X PBS. The resulting components will also result in a pH neutral isotonic salt hydrogel. The ending pH, osmo, and rheological properties are the same from Version 1.0 to Version 2.0.
Please reach out through the Contact Us tab if you have further questions. We appreciate working with you.
Gelin-S® is thiol-modified gelatin (denatured collagen) and is a component of the HyStem®-C, and HyStem-HP hydrogel kits. Most cells do not grow well on Gelin-S–only hydrogels. Instead, Gelin-S should be used in conjunction with Glycosil® (thiol-modified hyaluronic acid) or Heprasil® (thiol-modified hyaluronic acid with thiol-modified heparin). Reconstituted Gelin-S remains liquid at 15 to 37°C.
The gelatin used to make Gelin-S is from Type A Gelatin, Bloom 250, derived from porcine skin.
Gelin-S® (thiol-modified gelatin) is packaged in 5.0 mL vials containing 50mg. Vials are blanketed by nitrogen and under a slight vacuum.
Store Gelin-S in the original vial, unopened, at -20 °C for up to one year. Do not uncap the Gelin-S vials since they will crosslink in the presence of oxygen. Use a syringe and needle to add 1X PBS to the vial.
Note: It is recommended to reconstitute each vial in its entirety.
Product Q & A
The Buffer A is used to solubilize Glycosil, and is a buffered PBS. This will need to be adjusted, to ensure that the 2% Glycosil ends at a neutral pH.
Take the 10 mL bottle of Buffer A that comes with the 5 pack of glycosil 1 mL vials, and add an additional ~100-130 uL of sterile 1M NaOH to the Buffer A. Use this new Buffer A to solubilize the glycosil at 2% and you should end up with a pH of around 7.4-7.6.
We recommend using a Collagenase/Hyaluronidase mixture from Stemcell Technologies found here:
https://www.stemcell.com/products/collagenase-hyaluronidase.html
Catalog #07912
Yes, the Extralink concentration can be adjusted to tune the gelation speed and final gel strength of HyStem hydrogels, as shown below. The concentration represents the concentration of the extralink prior to mixing with Glycosil, not the final extralink concentration in the HyStem hydrogel. To clarify, we used a bulk bottle of Extralink https://advancedbiomatrix.com/extralink-pegda.html and solubilized that material at 0.5 - 4%. We then added that more concentrated Extralink to the other HyStem components in the same ratios as listed in the DFU.
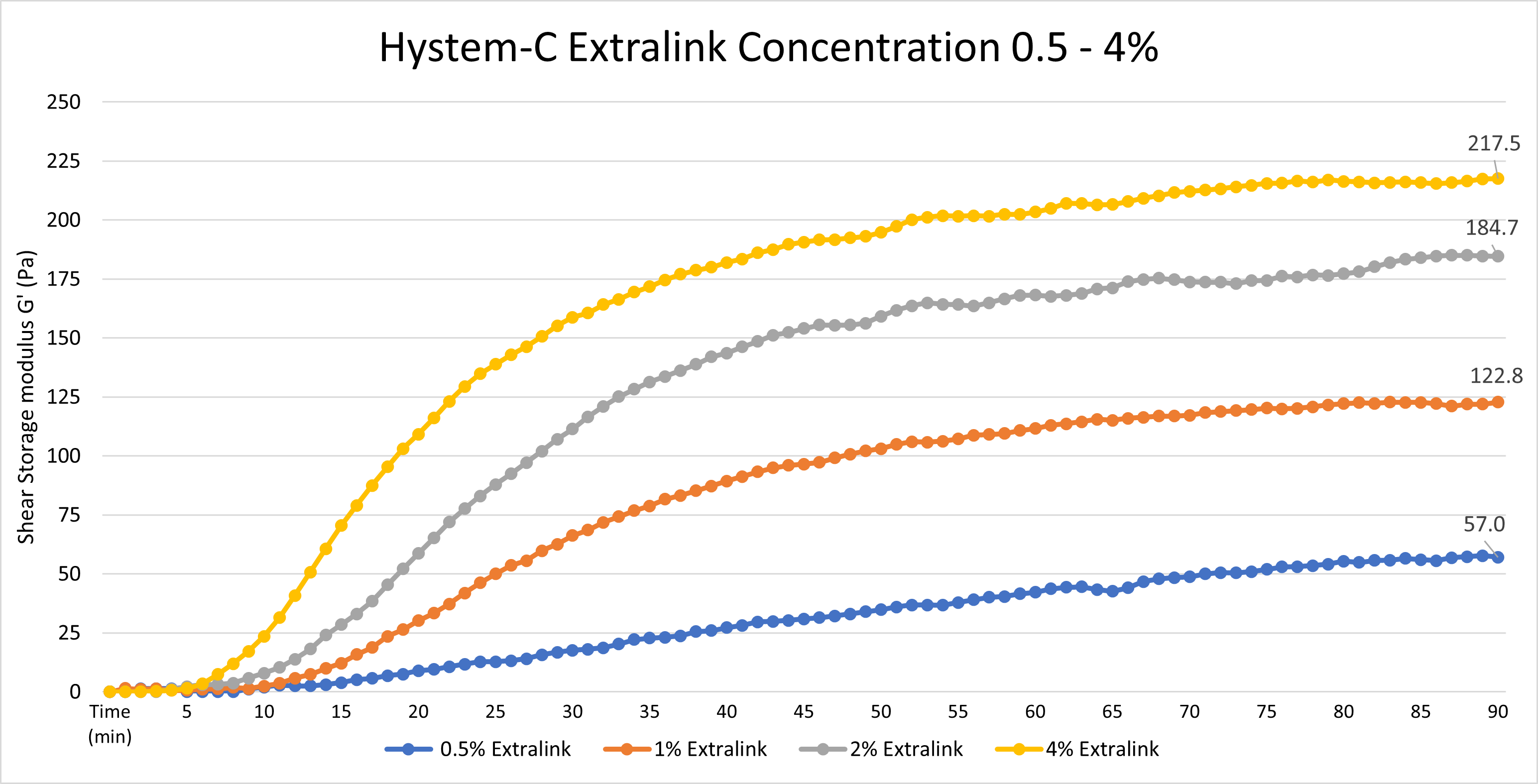
Data was collected using an Elastonsens rheometer from Rheolution: www.rheolution.com
Globular particles less than 75 kDa should be able to freely diffuse through a HyStem hydrogel.
When reconstituted using the reconstitution buffer, the pH of each HyStem component will be approximately 7.4-7.6.
One year from the date of receipt, if stored properly.
Gelin-S provides cellular attachment sites when incorporated in the hydrogel. Gelin-S is thiol-modified, denatured collagen I, derived from either bovine or porcine sources. Gelin-S is included in all HyStem-C and HyStem-HP kits.
Gelin-S has been thiol-modified in the same manner as the hyaluronan in Glycosil (or Heprasil), so that it covalently crosslinks with the Extralink in the HyStem hydrogels.
Yes. Peptides that contain a cysteine residue can be used. The cysteine residue must be present for the peptide to be covalently bonded to the hydrogel substrate.
Yes. ECM proteins, such as laminin, collagen, fibronectin, or vitronectin can be non-covalently incorporated into the hydrogel prior to crosslinking.
HyStem hydrogels and sponges differ in hydration and homogeneity. HyStem sponges are typically polymerized hydrogels that are subsequently freeze-dried. The resulting sponge is a fibrous, mesh network with pores and niches that enable cells to infiltrate and adhere. A true HyStem hydrogel is an encapsulating liquid that polymerizes around suspended cells in culture.
No. The compliance of the hydrogels is set by the amount of Extralink crosslinker added, the concentration of Glycosil (or Heprasil) and Gelin-S used, and the ratio of Glycosil (or Heprasil) to Gelin-S. Once this chemical structure of the hydrogel is fixed, it is not altered by prolonged exposure to cell culture medium.
HyStem sponges can be terminally sterilized by E-beam. HyStem hydrogels have not yet been validated for use with E-beam sterilization methods. HyStem hydrogels are not terminally sterilized by gamma irradiation.
Gelation time is affected by multiple aspects of the gel’s composition.
One way to change the gelation time of a hydrogel is to vary the amount of crosslinker used. Gels with a lower amount of Extralink crosslinker will have a longer gelation time than those with a higher amount of crosslinker. Changing the amount of crosslinker will produce slight changes in gelation time.
Gelation time can be dramatically changed by varying the Glycosil (or Heprasil) and Gelin-S concentrations. Concentrated solutions of Glycosil (or Heprasil) and Gelin-S will create a solution with a much shorter gelation time. This can easily be done by reconstituting the components in a smaller volume of DG Water. Alternatively, diluting these components in larger volumes of DG Water will dramatically increase the total time to form the hydrogel.
HyStem Hydrogels are virtually transparent and should not interfere with microscopy.
HyStem hydrogels may generate mild inflammation as part of the body’s natural healing process in response to injury. HyStem hydrogels do not trigger immune response when used in vivo. (These products are not for human use)
HyStem is degraded in vivo by matrix metalloproteinases (collagenases) and hyaluronidases.
Trypsin, Dipase, collagenase, and hyaluronidase have been used to help detach cells from the surface or from within HyStem hydrogels.
In general, the pore size for HyStem-C and HyStem-HP hydrogels is ~17 nm. In "Zarembinski, T., & Skardal, A. (2019). HyStem®: A Unique Clinical Grade Hydrogel for Present and Future Medical Applications. IntechOpen. doi: 10.5772/intechopen.81344" they use a value of <15.9 nm for pore size.
We routinely perform Ellmans test on our Thiolated Hyaluronic Acid. Our product falls between 0.55-0.75 umoles/mg, resulting in a degree of thiolation between 20-30%.
The approximate molecular weight of the thiolated hyaluronic acid is 100 kDa.
The hyaluronic acid has been thiol-modified on the carboxy groups. These active groups react with acrylate groups on PEGDA, resulting in a hydrogel.
We strongly recommend using the HyStem components the same day that they are solubilized, in order to minimize the chance of autocrosslinking. We cannot guarantee the quality of the product past the first day. If experiments need to be done across two or more days, we recommend leaving the solubilized components at room temperature overnight (capped, in the original vials). We do not recommend re-freezing the components once solubilized.
While we don’t have a specific protocol yet, we found this that might be helpful:
https://www.sciencedirect.com/science/article/pii/S0928493117342741
2.9. Gene expression analysis by qRT-PCR
For gene expression analysis 5 × 104 cells and 250 ng BMP-2 were encapsulated into the HyStem-HP® hydrogel and injected into the mPCL scaffold. The cells were cultured for 3 and 14 days prior to RNA isolation. Total RNA was isolated using Trizol (Life Technologies) according to the instructions provided by the supplier. RNA was quantified using a Nanodrop spectrophotometer. For cDNA synthesis, 2 μg of total RNA was used in the reverse transcription reaction. The reverse transcriptase M-MuLV (New England Biolabs) was used to synthesis cDNA according to the manufacturer's instruction. Briefly, 1 μg of total RNA was subjected to reverse transcription using M-MuLV reverse transcriptase. The initial denaturation and priming reaction consisted of 1 μl oligo dT primer (40 μM), 1 μl random hexamer primer (40 μM), 1 μl dNTP (10 mM), 1 μg RNA, and nuclease free water to a final volume of 16 μl. After denaturation at 70 °C and priming at 4 °C, complementary DNA was synthesised by the addition of 1 μl of M-MuLV Reverse Transcriptase (200 units/μl) and 2 μl of 10× RT buffer. The reaction mixture was incubated at 42 °C for 1 h followed by enzyme deactivation at 90 °C for 10 min. The prepared cDNA was stored at −20 °C until further analysis.
A collagenase/hyaluronidase mixture might also work well for extraction: https://www.stemcell.com/products/collagenase-hyaluronidase.html
Product Applications
Read our HyStem eBrochure Here
or
Click on the title of the desired protocol to learn more:
HyStem Hydrogel Stiffness/Rheology Curves
2D Cell Growth on HyStem Hydrogels
HyStem 3D Cell Encapsulation for Cell Delivery Applications Guide
HyStem 3D Cell Encapsulation in hydrogels using 96-well plates
HyStem 3D Cell Encapsulation in hydrogels using TC Inserts
Enzyme Digestion of HyStem Hydrogels for Recovery of Encapsulated Cells
Cell Recovery from Surface of HyStem Hydrogels
HyStem Gelation Time Variation
HyStem Stiffness Variation Protocol 12.5 mL Kit
HyStem Stiffness Variation Protocol 7.5 mL Kit
Product References
References for HyStem®:
Maloney, E. et al. Immersion Bioprinting of Tumor Organoids in Multi-Well Plates for Increasing Chemotherapy Screening Throughput. Micromachines 11, 208 (2020).
Gaetani, R., et al. (2015) Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials 61: 339-348. PMID: 17335875.
Prestwich, G.D., et al. (2007) 3-D culture in synthetic extracellular matrices: new tissue models for drug toxicology and cancer drug discovery. Adv Enzyme Regul 47: 196-207. PMID: 17335875.
Shu, X.Z., et al. (2006) Synthesis and evaluation of injectable, in situ crosslinkable synthetic extracellular matrices for tissue engineering. J Biomed Mater Res A 79: 901-912. PMID: 16941590.
Shu, X.Z., et al. (2003) Disulfide-crosslinked hyaluronan-gelatin hydrogel films: a covalent mimic of the extracellular matrix for in vitro cell growth. Biomaterials 24: 3825-3834. PMID: 12818555.
S. Cai, et al. (2005) Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor.Biomaterials, 26, 6054-6067.
D. B. Pike, et al. (2006) Heparin-regulated release of growth factors in vitro and angiogenic response in vivo to implanted hyaluronan hydrogels containing VEGF and bFGF. Biomaterials, 27, 5242–5251.
G. D. Prestwich, et al. (2007) 3-D Culture in Synthetic Extracellular Matrices: New Tissue Models for Drug Toxicology and Cancer Drug Discovery. invited, Adv. Enz. Res., in press (2007).
X. Z. Shu, et al, (2006) Synthesis and Evaluation of Injectable, In Situ Crosslinkable Synthetic Extracellular Matrices (sECMs) for Tissue Engineering. J. Biomed Mater. Res. A, 79A(4), 901-912.
Shu, X.Z., et al. (2004) In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials 25: 1339-1348. PMID: 14643608.
Mehra, T.D., et al. (2006) Molecular stenting with a crosslinked hyaluronan derivative inhibits collagen gel contraction. J Invest Dermatol 126: 2202-2209. PMID: 16741511.
Shu, X.Z., et al. (2004) Attachment and spreading of fibroblasts on an RGD peptide-modified injectable hyaluronan hydrogel. J Biomed Mater Res A 68: 365-375. PMID: 14704979.
Ghosh, K., et al. (2007) Cell adaptation to a physiologically relevant ECM mimic with different viscoelastic properties. Biomaterials 28: 671-679. PMID: 17049594.
Product Certificate of Analysis
No result for .
Product Videos
Product Disclaimer
This product is for R&D use only and is not intended for human or other uses. Please consult the Material Safety Data Sheet for information regarding hazards and safe handling practices.