-
Collagen
-
Type I - Atelocollagen
- PureCol® Solution, 3 mg/ml (bovine) #5005
- Nutragen® Solution, 6 mg/ml (bovine) #5010
- FibriCol® Solution, 10 mg/ml (bovine) #5133
- PureCol® EZ Gel, Solution, 5 mg/ml (bovine) #5074
- PureCol® Lyophilized, 15 mg (bovine) #5006
- VitroCol® Solution, 3 mg/ml (human) #5007
- VitroCol® Lyophilized, 15 mg (human) #5008
-
Type I - Telocollagen
- TeloCol®-3 Solution, 3 mg/ml (bovine) #5026
- TeloCol®-6 Solution, 6 mg/ml (bovine) #5225
- TeloCol®-10 Solution, 10 mg/ml (bovine) #5226
- RatCol™ for 2D and 3D, Solution, 4 mg/ml (rat) #5153
- RatCol™ High Concentration, Solution, 10 mg/ml (rat)
- RatCol™ lyophilized, 100 mg (rat)
- RatCol™ for Coatings, Solution, 4 mg/ml (rat) #5056
- Type I - Insoluble Collagen
- Type I - Bioinks
- Type II Collagen
- Type III Collagen
- Type IV Collagen
- Collagen Standard
-
PureCol® Collagen Coated Plates
- Collagen Coated T-25 Flasks #5029
- Collagen Coated 6-well Plates #5073
- Collagen Coated 12-well Plates #5439
- Collagen Coated 24-well Plates #5440
- Collagen Coated 48-well Plates #5181
- Collagen Coated 96-well Plates #5072
- Collagen Coated 384-well Plates #5380-5EA
- Collagen Coated 100 x 20 mm Dishes #5028
- MatTek Glass-Bottom Dishes
- MatTek Multi-Well Plates
- Collagen Scaffolds
- Collagen Imaging Reagents
-
Type I - Atelocollagen
- Tunable Stiffness
- CytoSoft™ Rigidity Plates
-
Bioprinting
- Support Slurry for FRESH Bioprinting
-
Bioinks for Extrusion Bioprinting
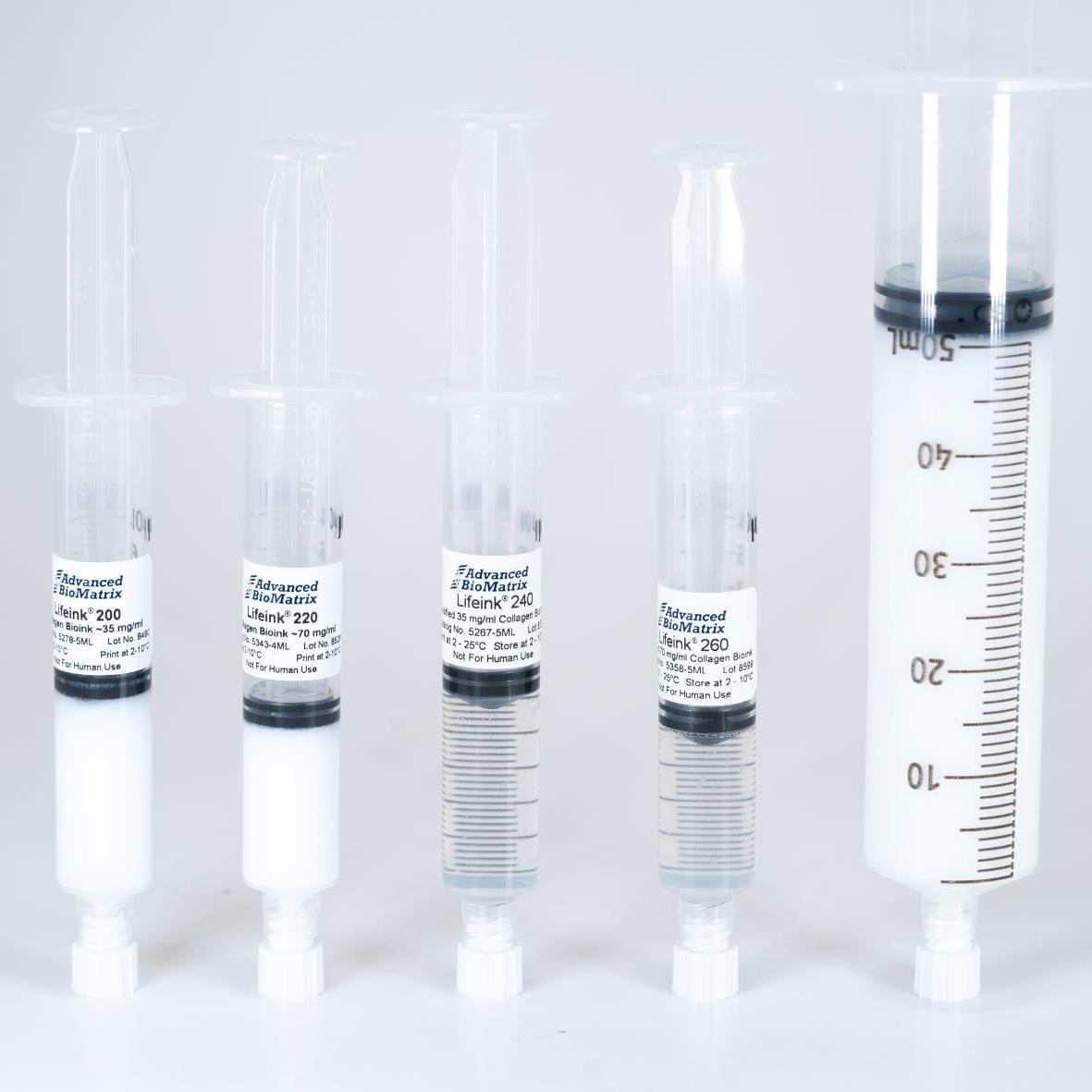
- Lifeink® 200 Collagen Bioink (35 mg/ml) #5278
- Lifeink® 220 Collagen Bioink (70 mg/ml) #5343
- Lifeink® 240 Acidic Collagen Bioink (35 mg/ml) #5267
- Lifeink® 260 Acidic Collagen Bioink (70 mg/ml) #5358
- GelMA Bioink
- GelMA A Bioink
- GelMA C Bioink
- Pluronic F-127 40% Sterile Solution
- GelMA 20% Sterile Solution
- Alginate 5% Sterile Solution
- Photoinitiators
- Bioinks for BIONOVA X
- Bioinks for Lumen X
- DLP Printing Consumables
-
Create Your Own Bioinks
- PhotoCol® Methacrylated Collagen
- PhotoGel® Methacrylated Gelatin 95% DS
- PhotoGel® Methacrylated Gelatin 50% DS
- PhotoHA®-Stiff Methacrylated Hyaluronic Acid
- PhotoHA®-Soft Methacrylated Hyaluronic Acid
- PhotoAlginate® Methacrylated Alginate
- PhotoDextran® Methacrylated Dextran
- PEGDA (Various Molecular Weights)
- Silk Fibroin, Solution
- PhotoSericin® Methacrylated Sericin
- Bioprinters
-
3D Hydrogels
- Thermoreversible Hydrogel
- Silk Fibroin
-
Type I Collagen for 3D Hydrogels
- PureCol® Solution, 3 mg/ml (bovine) #5005
- Nutragen® Solution, 6 mg/ml (bovine) #5010
- FibriCol® Solution, 10 mg/ml (bovine) #5133
- PureCol® EZ Gel, Solution, 5 mg/ml (bovine) #5074
- VitroCol® Solution, 3 mg/ml (human) #5007
- TeloCol®-3 Solution, 3 mg/ml (bovine) #5026
- TeloCol®-6 Solution, 6 mg/ml (bovine) #5225
- TeloCol®-10 Solution, 10 mg/ml (bovine) #5226
- RatCol® for 3D gels, Solution, 4 mg/ml (rat) #5153
- HyStem® Thiolated Hyaluronic Acid
- Methacrylated Collagen
- Methacrylated Gelatin
- Methacrylated Hyaluronic Acid
- Diacrylates
- Collagen Sponges
- Methacrylated Polysaccharides
- Spheroids and Organoids
- Extracellular Matrices
- HyStem / Hyaluronic Acid
-
Adhesion Peptides / Proteins
-
Recombinant Adhesion Proteins
- CD2, 0.5 mg/ml #5086
- CDH3, 0.5 mg/ml #5124
- CDH13, 0.5 mg/ml #5125
- CD14, 0.5 mg/ml #5089
- CDH18, 0.5 mg/ml #5090
- CD40, 0.5 mg/ml #5093
- CD86, 0.5 mg/ml #5096
- CD164, 0.5 mg/ml #5100
- CD270, 0.5 mg/ml #5127
- CD274, 0.5 mg/ml #5126
- CD276, 0.5 mg/ml #5123
- E-Cadherin (CD324), 0.5 mg/ml #5085
- ICAM2, 0.5 mg/ml #5107
- Adhesion Peptides
- Collagen Hybridizing Peptides
-
Recombinant Adhesion Proteins
- Reagents
- Assays
Lifeink® 240
35 mg/ml Acidic Type I Collagen Bioink
Catalog #5267
Lifeink® 240
35 mg/ml Acidic Type I Collagen Bioink
Catalog #5267
Lifeink® 240 is a 35 mg/ml type I collagen bioink for extrusion based FRESH 3D bioprinting. Lifeink® 240 comes with acidic pH and when FRESH printed into LifeSupport® yields a neutralized, high resolution collagen scaffold.
Product Description
Lifeink® 240 is an acidic Type I collagen bioink at a concentration of 35 mg/ml for extrusion-based 3D bioprinting. The product is a highly concentrated acidified collagen solution intended to be extrusion printed employing FRESH bioprinting (see LifeSupport® Catalog No. 5244-8GM).
Lifeink® 240 produces printed structures with a high print resolution and good mechanical strength. The product is formulated in an acidic saline buffer solution. Once the collagen is printed into LifeSupport®, the pH and salts concentration of the printed structure become physiological. Cells can then be seeded onto the printed structure allowing for cell adherence and cellular remodeling of the 3D bioprinted structure.
| Parameter, Testing, and Method | Lifeink® 240 #5267 |
| Sterilization Method | Filtration, Aseptic Processing |
| Sterility - USP modified | No growth |
| Form | Clear Viscous Solution |
| Package Size | 5 mL |
| Storage Temperature | 2-8°C |
| Print Temperature | 2-25°C |
| Shelf Life | Minimum of 6 months from date of receipt |
| Endotoxin - LAL | < 10.0 EU/mL |
| pH | 3.0-5.0 |
|
Osmolality (mOsmo H2O/kg) |
450-700 |
|
Source Material |
Bovine Collagen |
|
Electrophoretic Pattern - Coomassie Blue |
Characteristic |
|
Collagen Concentration - Biuret |
30-40 mg/mL |
|
Collagen Purity - Silver Staining |
>99% |
|
Continuous Flow Extrusion |
Pass |
Directions for Use
Download the full PDF version or continue reading below:
Note: Employ aseptic practices to maintain the sterility of the product throughout the preparation and handling of the collagen and other solutions.
Note: Ensure that NO bubbles enter the system if mixing other materials. Bubbles in the system while mixing will turn your ink into a foam-like material.
Note: Cells should not be added directly to the Lifeink® 240 bioink since it is an acidic formulation.
Addition of other components to Lifeink® 240 Bioink:
Note: Components other than cells can be added to the Lifeink® 240 bioink before bioprinting as long as these components are compatible with acidic pH conditions.
- To mix in additives to the bioink, add the additives to a secondary syringe as demonstrated in the video below:
Note: The video shows cells being added to the bioink. Other materials can be added to the Lifeink® 240 bioink in lieu of cells. Cells should NOT be added directly to this bioink. For cellular bioprinting, use Lifeink® 200 #5278-5ML.
Note: For best results, add no more than 5 mL of other components per 5 mL of collagen bioink. Use a similar ratio for smaller volumes.
- Place sterile coupler on the end of the syringe with the bioink additives.
- Slowly push plunger in until solution forms a slight external meniscus above the end of the coupler on the syringe.
- Remove cap from the syringe with collagen and slowly push plunger in until collagen forms a slight external meniscus above the end of the syringe.
- Couple the syringe with additives to the syringe with collagen. (Ensure that there are no air bubbles in the system. The “external meniscus” on both syringes helps ensure that there are no air bubbles introduced).
- Slowly push plungers back and forth ~40 times to ensure thorough mixing. End with all of the material in the syringe to be used for printing.
- The bioink with other components is now ready for extrusion 3D bioprinters.
Note: For pneumatic printers, transfer the collagen into an appropriate syringe using the coupler. The new syringe should have the seal inserted, but the plunger removed. Centrifuge the syringe at 2000 RPM for 1 minute after transferring the collagen to remove any air bubbles.
General Printing Notes:
- To use a smaller volume of collagen, simply transfer the desired amount of collagen to another syringe, using the provided sterile coupler. To remove the air from the new syringe, you can do either of the following:
-
- Centrifuge the syringe (capped) with the cap pointing up to cause the air to accumulate at the cap. Evacuate the air.
- Centrifuge the syringe (capped) with the cap pointing down, and then use a hemostat to squeeze the syringe while pushing the plunger to allow the air to escape.
Removing air with a hemostat video
- When printing with FRESH gelatin slurry, allow the final printed structure to incubate at 37°C for 30 to 60 minutes and then replace the gelatin with media.
- Avoid bubbles.
For more directions on FRESH printing, please visit our LifeSupport® Directions for Use.
Product Applications
Read our Bioprinting eBrochure Here
Bioprinted collagen constructs can be secondarily crosslinked using photocrosslinking and the ruthenium/sodium persulfate system. Read the recent publication outlining this process here.
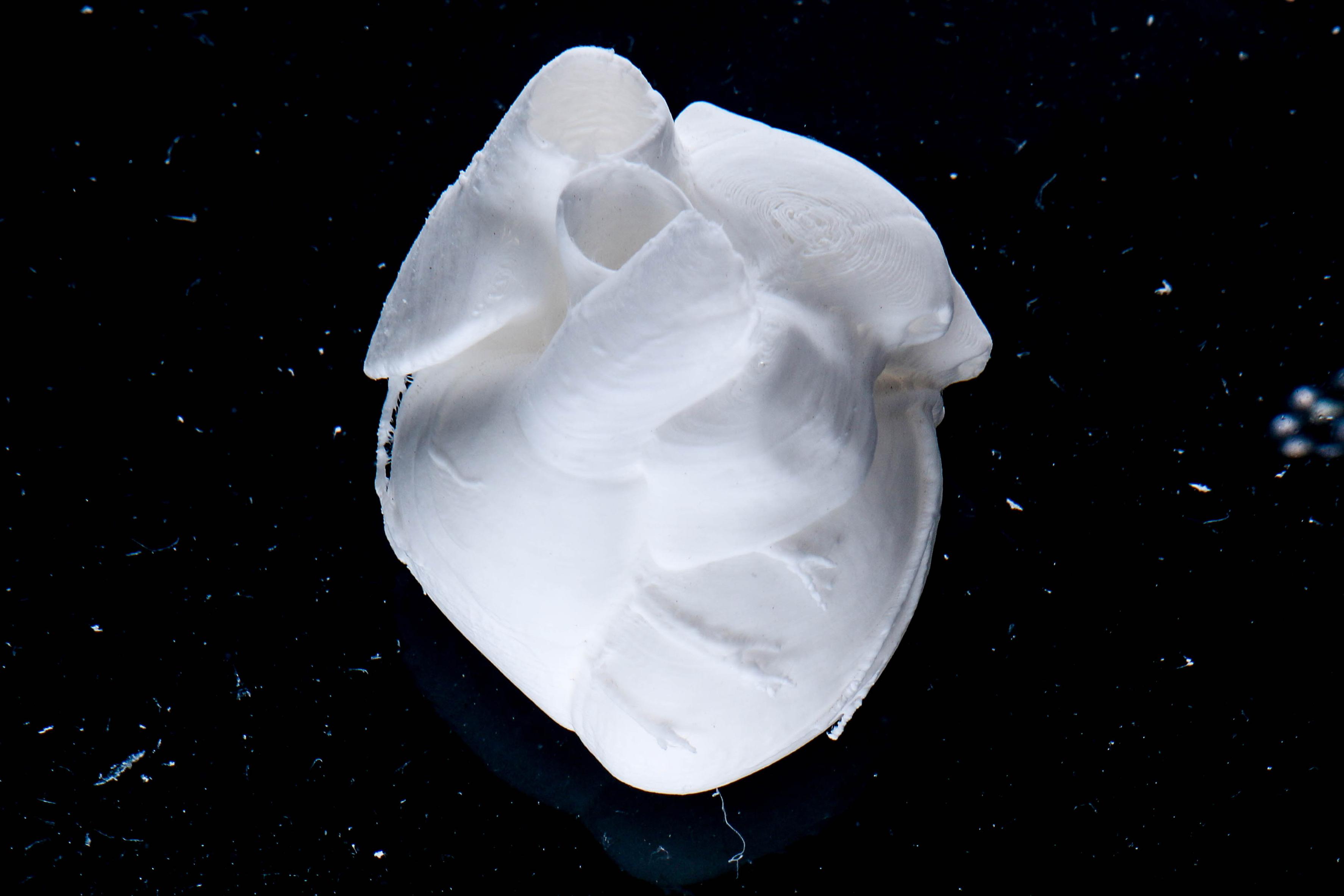
Product Bioprints
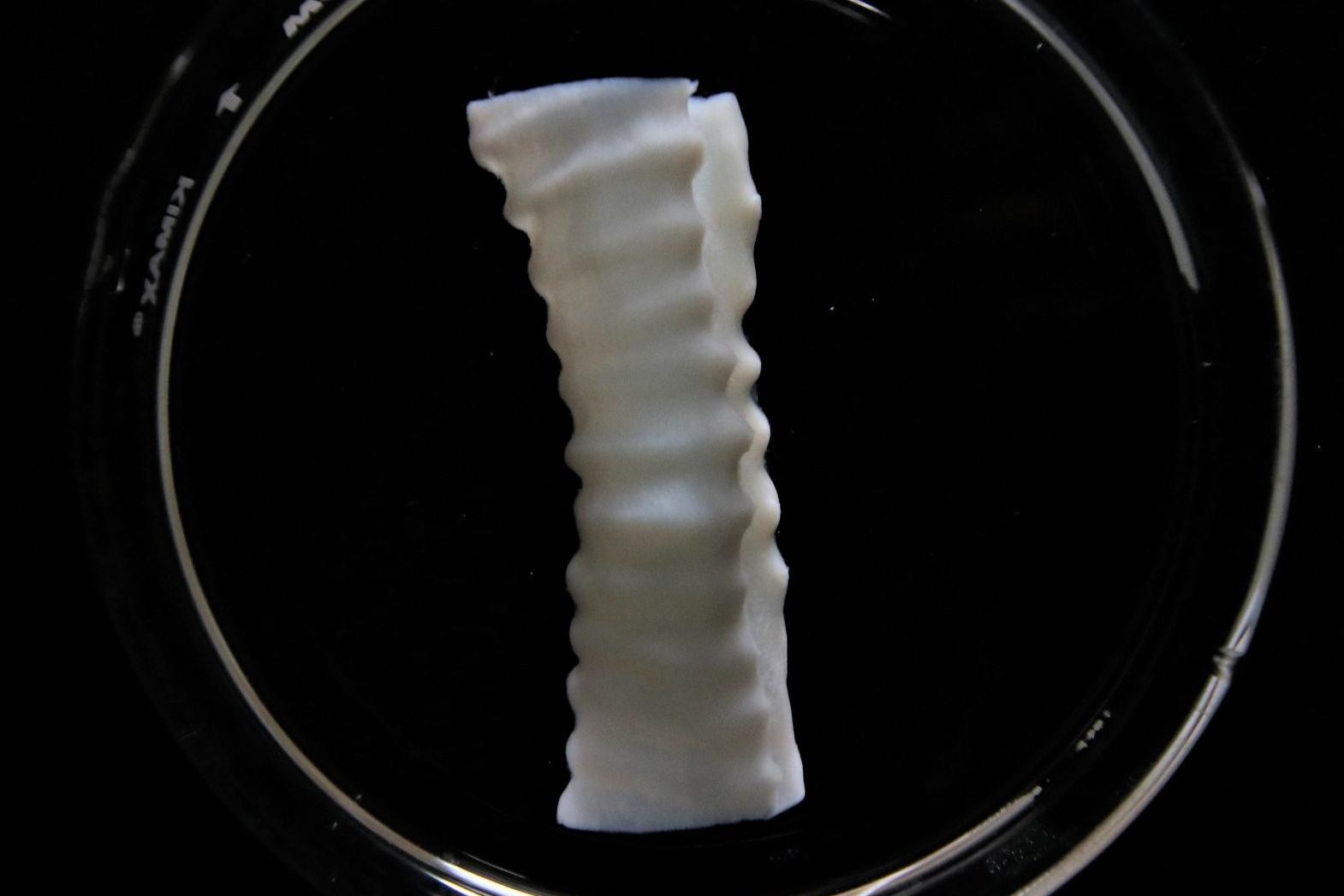
Heart
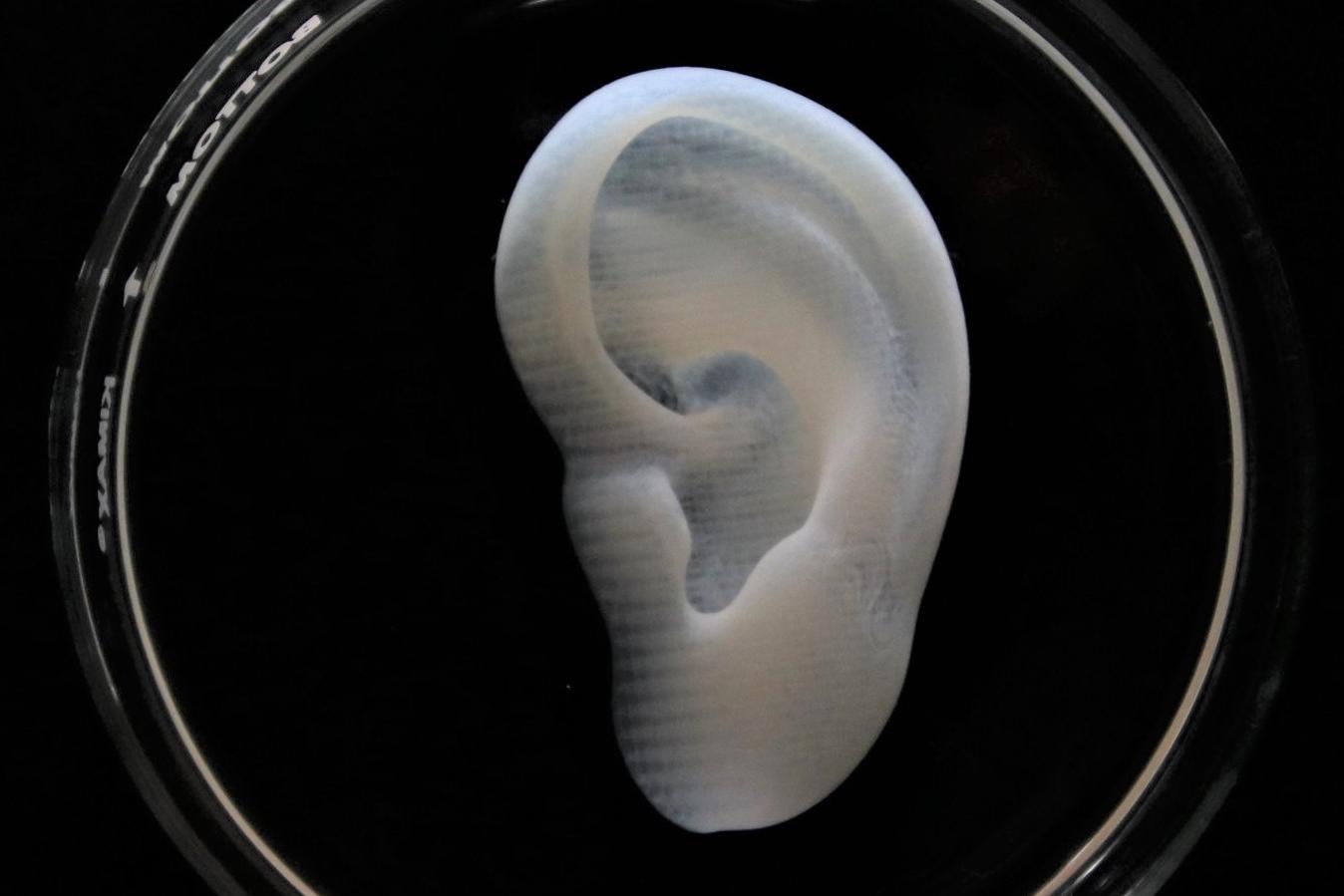
3D bioprinted collagen ear
3D bioprinted collagen ear with Lifeink 240 and LifeSupport
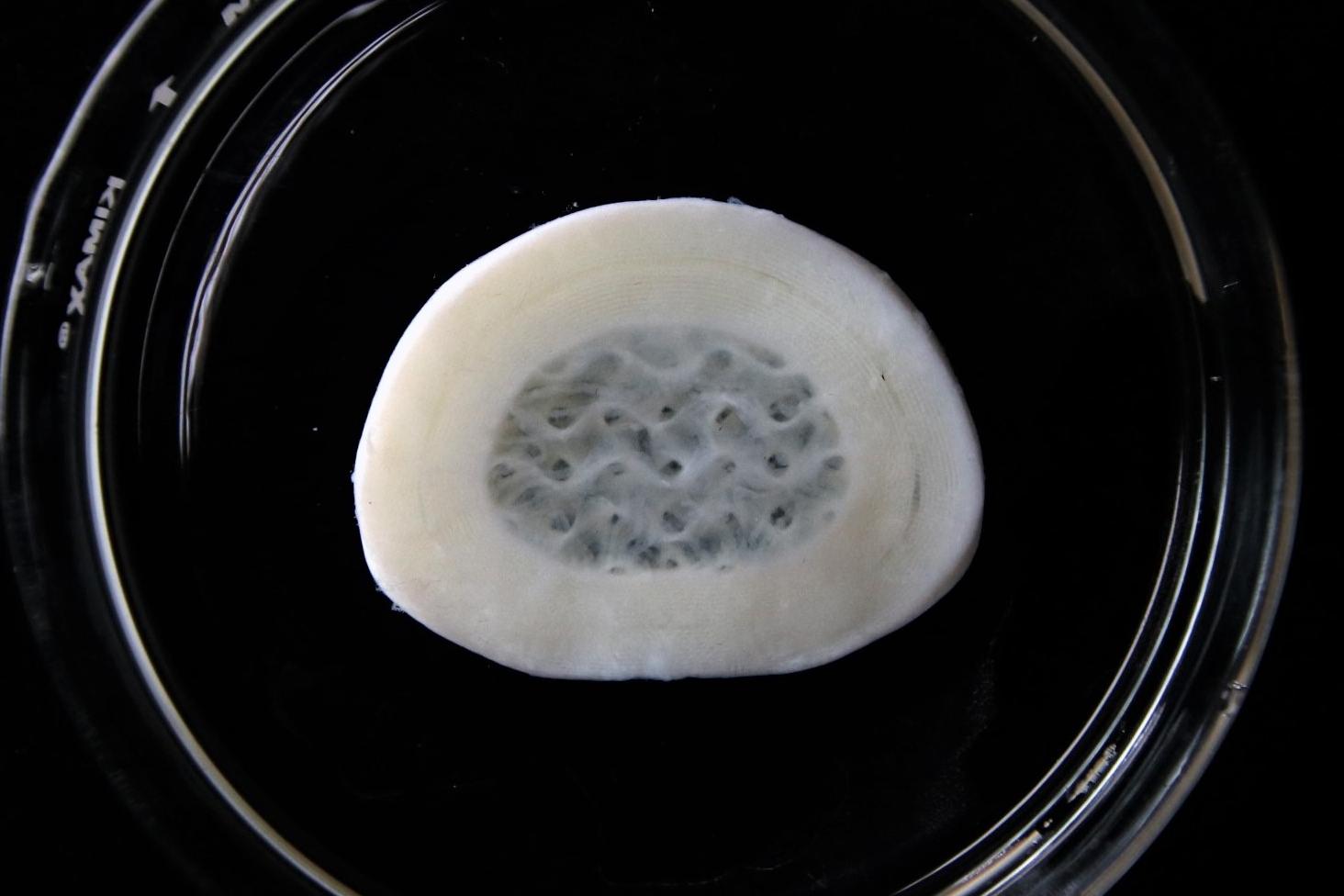
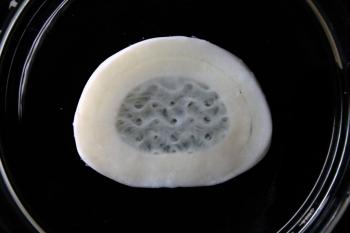
3D bioprinted intervertebral disc
3D bioprinted intervertebral disc with Lifeink 240 and LifeSupport
3D bioprinted Trachea with Lifeink 240 and LifeSupport
3D bioprinted Trachea with Lifeink 240 and LifeSupport
Product References
References for Lifeink® collagen bioinks:
Lan, X. et al. In vitro maturation and in vivo stability of bioprinted human nasal cartilage. Journal of Tissue Engineering 13, 204173142210863 (2022).
Stocco, T. D., Moreira Silva, M. C., Corat, M. A., Gonçalves Lima, G. & Lobo, A. O. Towards bioinspired meniscus-regenerative scaffolds: Engineering A novel 3D bioprinted patient-specific construct reinforced by biomimetically aligned nanofibers. International Journal of Nanomedicine Volume 17, 1111–1124 (2022).
Lee, A. et al. 3D bioprinting of collagen to rebuild components of the human heart. Science 365, 482–487 (2019).
Maxson, Eva L., et al. "In vivo remodeling of a 3D-Bioprinted tissue engineered heart valve scaffold." Bioprinting (2019): e00059.
Filardo, G. et al.Patient-specific meniscus prototype based on 3D bioprinting of human cell-laden scaffold. Bone & Joint Research 8,101–106 (2019).
Schmitt, T. Analysis and Classification of 3-D Printed Collagen-Bioglass Matrices for Cellular Growth Utilizing Artificial Neural Networks. University Thesis (2018).
Balakhovsky, Y. M., Ostrovskiy, A. Y. & Khesuani, Y. D. Emerging Business Models Toward Commercialization of Bioprinting Technology. 3D Printing and Biofabrication1–22 (2017). doi:10.1007/978-3-319-40498-1_25-1
Fox, S. et al. A simplified fabrication technique for cellularized high-collagen dermal equivalents. Biomedical Materials14,041001 (2019).
Lin, H.-H., Chao, P.-H. G., Tai, W.-C. & Chang, P.-C. 3D-printed collagen-based waveform microfibrous scaffold for periodontal ligament reconstruction. International Journal of Molecular Sciences 22, 7725 (2021).
Engberg, A., Stelzl, C., Eriksson, O., O’Callaghan, P. & Kreuger, J. An open source extrusion bioprinter based on the E3D motion system and Tool Changer to enable fresh and multimaterial bioprinting. Scientific Reports 11, (2021).
Tashman, J., et al. "In Situ Volumetric Imaging and Analysis of FRESH 3D Bioprinted Constructs Using Optical Coherence Tomography." BioRxiv. (2021)
Product Certificate of Analysis
No result for .
Product Videos
Product Disclaimer
This product is for R&D use only and is not intended for human or other uses. Please consult the Material Safety Data Sheet for information regarding hazards and safe handling practices.