-
Collagen
-
Type I - Atelocollagen
- PureCol® Solution, 3 mg/ml (bovine) #5005
- Nutragen® Solution, 6 mg/ml (bovine) #5010
- FibriCol® Solution, 10 mg/ml (bovine) #5133
- PureCol® EZ Gel, Solution, 5 mg/ml (bovine) #5074
- PureCol® Lyophilized, 15 mg (bovine) #5006
- VitroCol® Solution, 3 mg/ml (human) #5007
- VitroCol® Lyophilized, 15 mg (human) #5008
-
Type I - Telocollagen
- TeloCol®-3 Solution, 3 mg/ml (bovine) #5026
- TeloCol®-6 Solution, 6 mg/ml (bovine) #5225
- TeloCol®-10 Solution, 10 mg/ml (bovine) #5226
- RatCol™ for 2D and 3D, Solution, 4 mg/ml (rat) #5153
- RatCol™ High Concentration, Solution, 10 mg/ml (rat)
- RatCol™ lyophilized, 100 mg (rat)
- RatCol™ for Coatings, Solution, 4 mg/ml (rat) #5056
- Type I - Insoluble Collagen
- Type I - Bioinks
- Type II Collagen
- Type III Collagen
- Type IV Collagen
- Collagen Standard
-
PureCol® Collagen Coated Plates
- Collagen Coated T-25 Flasks #5029
- Collagen Coated 6-well Plates #5073
- Collagen Coated 12-well Plates #5439
- Collagen Coated 24-well Plates #5440
- Collagen Coated 48-well Plates #5181
- Collagen Coated 96-well Plates #5072
- Collagen Coated 384-well Plates #5380-5EA
- Collagen Coated 100 x 20 mm Dishes #5028
- MatTek Glass-Bottom Dishes
- MatTek Multi-Well Plates
- Collagen Scaffolds
- Collagen Imaging Reagents
-
Type I - Atelocollagen
- Tunable Stiffness
- CytoSoft™ Rigidity Plates
-
Bioprinting
- Support Slurry for FRESH Bioprinting
-
Bioinks for Extrusion Bioprinting
- Lifeink® 200 Collagen Bioink (35 mg/ml) #5278
- Lifeink® 220 Collagen Bioink (70 mg/ml) #5343
- Lifeink® 240 Acidic Collagen Bioink (35 mg/ml) #5267
- Lifeink® 260 Acidic Collagen Bioink (70 mg/ml) #5358
- GelMA Bioink
- GelMA A Bioink
- GelMA C Bioink
- Pluronic F-127 40% Sterile Solution
- GelMA 20% Sterile Solution
- Alginate 5% Sterile Solution
- Photoinitiators
- Bioinks for BIONOVA X
- Bioinks for Lumen X
- DLP Printing Consumables
-
Create Your Own Bioinks
- PhotoCol® Methacrylated Collagen
- PhotoGel® Methacrylated Gelatin 95% DS
- PhotoGel® Methacrylated Gelatin 50% DS
- PhotoHA®-Stiff Methacrylated Hyaluronic Acid
- PhotoHA®-Soft Methacrylated Hyaluronic Acid
- PhotoAlginate® Methacrylated Alginate
- PhotoDextran® Methacrylated Dextran
- PEGDA (Various Molecular Weights)
- Silk Fibroin, Solution
- PhotoSericin® Methacrylated Sericin
- Bioprinters
-
3D Hydrogels
- Thermoreversible Hydrogel
- Silk Fibroin
-
Type I Collagen for 3D Hydrogels
- PureCol® Solution, 3 mg/ml (bovine) #5005
- Nutragen® Solution, 6 mg/ml (bovine) #5010
- FibriCol® Solution, 10 mg/ml (bovine) #5133
- PureCol® EZ Gel, Solution, 5 mg/ml (bovine) #5074
- VitroCol® Solution, 3 mg/ml (human) #5007
- TeloCol®-3 Solution, 3 mg/ml (bovine) #5026
- TeloCol®-6 Solution, 6 mg/ml (bovine) #5225
- TeloCol®-10 Solution, 10 mg/ml (bovine) #5226
- RatCol® for 3D gels, Solution, 4 mg/ml (rat) #5153
- HyStem® Thiolated Hyaluronic Acid
- Methacrylated Collagen
- Methacrylated Gelatin
- Methacrylated Hyaluronic Acid
- Diacrylates
- Collagen Sponges
- Methacrylated Polysaccharides
- Spheroids and Organoids
- Extracellular Matrices
- HyStem / Hyaluronic Acid
-
Adhesion Peptides / Proteins
-
Recombinant Adhesion Proteins
- CD2, 0.5 mg/ml #5086
- CDH3, 0.5 mg/ml #5124
- CDH13, 0.5 mg/ml #5125
- CD14, 0.5 mg/ml #5089
- CDH18, 0.5 mg/ml #5090
- CD40, 0.5 mg/ml #5093
- CD86, 0.5 mg/ml #5096
- CD164, 0.5 mg/ml #5100
- CD270, 0.5 mg/ml #5127
- CD274, 0.5 mg/ml #5126
- CD276, 0.5 mg/ml #5123
- E-Cadherin (CD324), 0.5 mg/ml #5085
- ICAM2, 0.5 mg/ml #5107
- Adhesion Peptides
- Collagen Hybridizing Peptides
-
Recombinant Adhesion Proteins
- Reagents
- Assays
CytoSoft™ T-25 Flasks
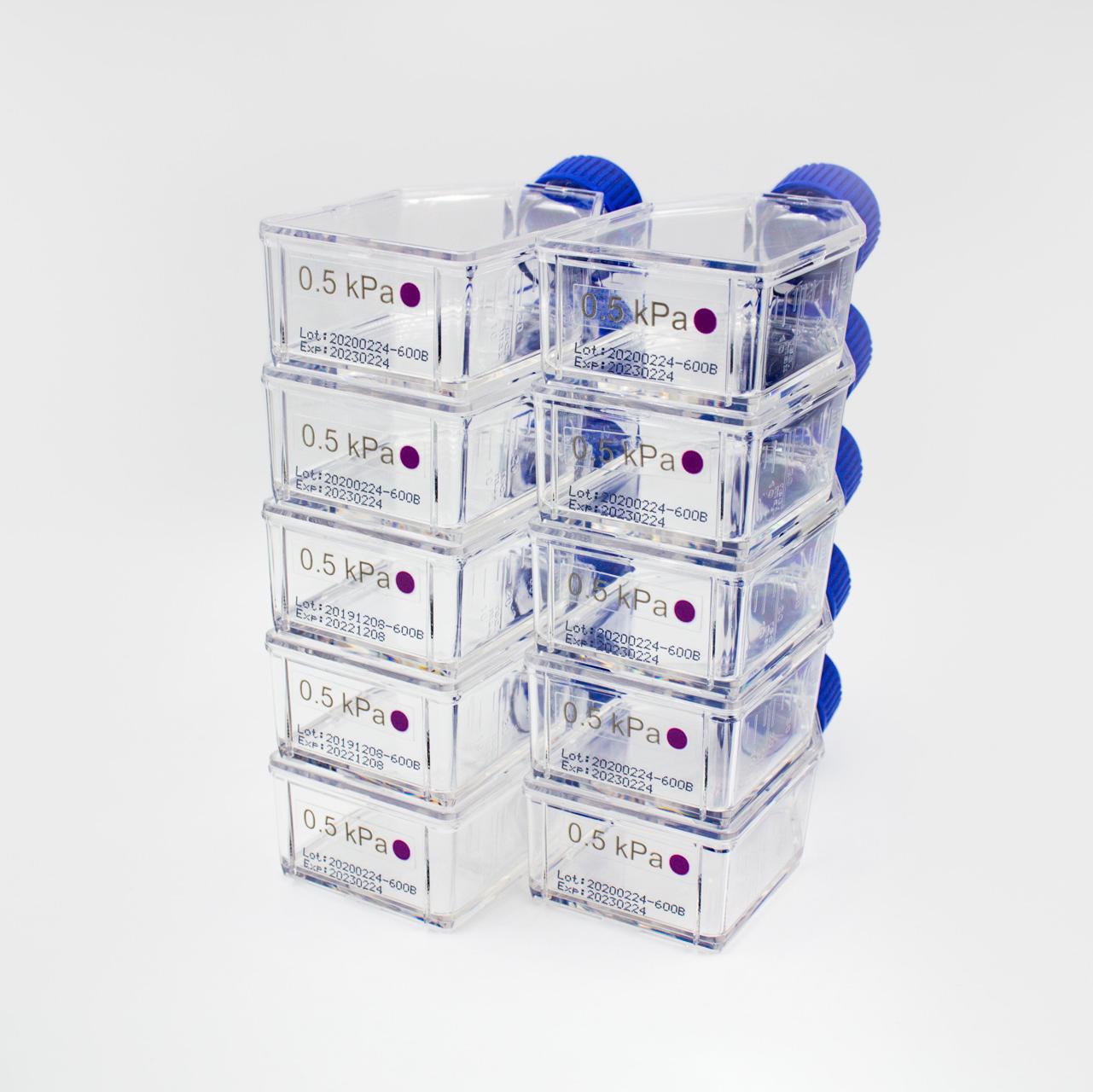
10 Flasks of a Specific Elastic Modulus (0.2, 0.5, 2, 8, 16, 32 or 64 kPa)
Catalog # (Various Options - Choose the Stiffness Below)
CytoSoft™ T-25 Flasks
10 Flasks of a Specific Elastic Modulus (0.2, 0.5, 2, 8, 16, 32 or 64 kPa)
Catalog # (Various Options - Choose the Stiffness Below)
CytoSoft™ products provide a tool to culture cells on PDMS substrates with various rigidity covering a broad physiological range, including 0.2, 0.5, 2, 8, 16, 32, and 64 kPa. This CytoSoft™ product comes with 10 standard T-25 flasks with substrates of a chosen stiffness (selected below).
Product Description
This CytoSoft™ T-25 Flask product has a defined elastic modulus (see certificate of analysis) in the bottom of a standard T-25 Flask. The thickness of the silicone gel is uniform with a ~0.5 mm thick layer of silicone in each flask that is fully compatible with mammalian cell cultures. The silicone gels are activated and ready to bind to a purified ECM, such as PureCol® type I collagen (#5005) prior to cell addition. The plates are packaged sterilized, and provided with 10 flasks per package.
The rigidity of the substrate to which cells adhere can have a profound effect on cell morphology and gene expression. CytoSoft™ products provide a tool to culture cells on substrates with various rigidities covering a broad physiological range. On the bottom of each flask, there is a thin layer of specially formulated biocompatible silicone, whose elastic modulus (rigidity) is carefully measured and certified. The surfaces of the gels in CytoSoft™ products are functionalized to form covalent bonds with amines on proteins. This chemical functionalization is stable and the reaction does not require a catalyst, facilitating the coating of the gel surfaces with matrix proteins and plating cells.
The silicone substrates of CytoSoft™ products are optically clear and have a low auto-florescence. The layer of silicone in each flask is firmly bonded to the bottom of the flask. Unlike hydrogels (such as polyacrylamide gels), silicone gels are not susceptible to hydrolysis, do not dry nor swell, are resilient and resistant to tearing or cracking, and their elastic moduli (rigidities) remain nearly unchanged during extended storage periods.
CytoSoft™ products accommodate the harvesting of cells using enzymes such as trypsin and collagenase. There is no biochemical breakdown of the substrate during or after enzyme treatment, and there are no residuals of the substrate in the sample retrieved from a CytoSoft™ plate.
| Parameter, Testing, and Method | CytoSoft™ T-25 Flasks |
| Sterilization Method | Ozone |
| Plate Size | T-25 |
| Quantity per Package | 10 Flasks |
| Rigidty (Elastic Modulus) | 0.2, 0.5, 2, 8, 16, 32, 64 kPa (exact values provided on the CofA) |
| Storage Temperature | Room Temperature |
| Shelf Life | Minimum of 6 months from date of receipt |
| Plate Surface Material | Functionalized Silicone |
|
Growth Area per Well |
25 cm2 |
|
Cell Attachment Assay |
Pass |
Directions for Use
Coating Procedure
Note: Use these recommendations as guidelines to determine the optimal coating conditions for your culture system.
Remove the CytoSoft™ product from the protective sleeve in a sterile hood.
- Prepare extracellular matrix material by neutralizing in amine-free buffer pH 7.4 to 7.9 (such as 1X DPBS). We do not recommend using gelatin as your ECM protein.
Note: Pre-warm the coating solution to approximately room temperature before use.
- Dilute as needed, and dispense 10ml of solution into each flask to coat the surface.
Note: Recommended dilution for PureCol® Type I collagen is 1:30 (~100 µg/ml).
Note: The hydrophobic surface requires larger volumes to cover the surface than do conventional plastic dishes
- Incubate ECM coated CytoSoft® at room temperature, covered for 0.5-1 hour.
- After incubation, aspirate any remaining material and rinse coated surfaces immediately two times with culture medium or PBS. Leave about 5-10 ml of medium per flask to keep surface covered.
Note: Do not allow the CytoSoft® surface to become dry once the surface has been wetted.
5. Coated surfaces are ready for use.
For T-Flasks, allow 1 full day for cell attachment before media change, and keep the flasks level. If the flasks are tilted or moved too soon, the media can shear the cells off the surface.
Standard harvesting procedures used for removing cells from cultureware can be employed for harvesting cells from the CytoSoft® product including use of trypsin, Accutase® and non-enzymatic cell detachment solutions.
Product Q & A
|
Product |
Catalog # |
Starting Volume |
Dilution Factor |
Ending Volume |
Final Working Concentration |
|
PureCol® Type I collagen |
#5005-100ML |
100 mL |
1:30 |
3000 mL |
100 µg/mL |
|
Fibronectin |
#5050-1MG |
2 mL |
1:10 |
20 mL |
50 µg/mL |
|
Vitronectin |
#5051-0.1MG |
0.2 mL |
1:50 |
10 mL |
10 µg/mL |
|
Type IV Collagen |
#5022-5MG |
5 mL |
1:20 |
100 mL |
50 µg/mL |
| Publication | Coating material | Cells |
| Astrogliosis in a Dish: Substrate Stiffness Induces Astrogliosis in Primary Rat Astrocytes | Poly-L-Lysine | Primary Rat Astrocytes |
| Cellular Microenvironment Stiffness Regulates Eicosanoid Production and Signaling Pathways | RatCol Type I Collagen | Human Pulmonary Fibroblasts |
| Deciphering the role of substrate stiffness to enhance internalization efficiency of plasmid DNA in stem cells using lipid-based nanocarriers | High Purity Gelatin A, 1% in PBS | Human Adipose-Derived Stem Cells |
| Dependence of Membrane Tether Strength on Substrate Rigidity Probed by Single-Cell Force Spectroscopy | Not Reported | Human Breast Cancer Cells, Human Cervical Cancer Cells, Human Lung Cancer Cells |
| High Refractive Index Silicone Gels for Simultaneous Total Internal Reflection Fluorescence and Traaction Force Microscopy of Adherent Cells | Fibronectin | Human Umbilical Vein Endothelial Cells |
| Fibroblast Polarization is a Matrix-Rigidity-Dependent Process Controlled by Focal Adhesion Mechanosensing | Fibronectin | Human Foreskin Fibroblasts |
| Matrix Elasticity, Replicative Senescence and DNA Methylation Patterns of Mesenchymal Stem Cells | Not Reported | Mesenchymal Stem Cells |
| Micropatterned Silicone Elastomer Substrates for High Resolution Analysis of Cellular Force Patterns | Fibronectin | Myocytes, Cardiac Fibroblasts |
| Migration of Glial Cells Differentiated from Neurosphere-Forming Neural Stem/Progenitor Cells Depends on the Stiffness of the Chemically Cross-Linked Collagen Gel Substrate | Type I Collagen | Glial Cells, Neural Cells |
| Myosin II Governs Intracellular Pressure and Traction by Distrinct Tropomyosin-Dependent Mechanisms | Not Reported | Primary Human Dermal Fibroblasts |
| Periostin and Matrix Stiffness Combine to Regulate Myofibroblast Differentiation and Fibronectin Synthesis During Palatal Healing | PureCol Type I Collagen | Murine Primary Palatal Fibroblasts |
| Rigid Substrate Induces Esophageal Smooth Muscle Hypertrophy and EoE Fibrotic Gene Expression | Not Reported | Longitudinal Smooth Muscle Cells |
| Rigidity of Silicone Substrates Controls Cell Spreading and Stem Cell Differentiation | PureCol Type I Collagen | Human Mesenchymal Stem Cells |
| Spinal Cord Injury Results in Chronic Mechanical Stiffening | Poly-D-Lysine | Neural Progenitor Cells, Astrocytes |
| Stiffness of the Extracellular Matrix: A Regulator of Prostaglandins in Pulmonary Fibrosis | Not Reported | Human Lung Fibroblasts |
| TGF-B1 Induces Amoeboid-to-Mesenchymal Transition of CD44 Oral Squamous Cell Carcinoma Cells via MiR-422a Downregulation Through ERK Activation and Cofilin-1 Phosphorylation | Laminin-332 | Human Oral Squamous Cell Carcinoma Cells |
| TGF-B-Induced Activation of Conjunctival Fibroblasts is Modulated by FGF-2 and Substratum Stiffness | PureCol Type I Collagen |
Human Conjunctival Fibroblasts |
| Alpha-Parvin Defines a Specific Integrin Adhesome to Maintain the Glomerular Filtration Barrier | Type IV Collagen |
Podocytes |
| L-Plastin Enhances NLRP3 Inflammasome Assembly and Bleomycin-Induced Lung Fibrosis | Collagen I |
Alveolar Macrophages |
|
The Transcription Factor PREP1 Regulates Nuclear Stiffness, the Expression of LINC Complex Proteins and Mechanotransduction |
Not Reported |
HeLa and Mesenchymal Stromal Cells |
|
Substrate Stiffness Induces Neutrophil Extracellular Trap (NET) Formation Through Focal Adhesion Kinase Activation |
Peptite-2000 (RGD), Collagen, Fibronectin, Laminin |
Neutraphils |
We recommend a 60X objective for the imaging plates.
We actually use the method outlined in this journal:
http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0025534
To summarize, we have a layer of gold particles on the bottom of a specially prepared slide. We have the silicone on top of those gold particles, and then we add more gold particles on top of the silicone. We then centrifuge the sample at various speeds and measure the relative displacement/movement of the top beads (on the silicone), compared to the bottom beads (attached to the glass bottom). We then use a formula to calculate the youngs modulus of the silicone for that particular sample.
Using prewarmed media will decrease gas solubility and help prevent bubbles on the surface of the silicone.
The silicone gel can crack if the surface becomes dry.
No. Cell matrix proteins are attached to the surface of the gel via covalent bonding. It is difficult to form a new ECM layer after cell detachment. There are no longer any reactive groups on the surface of the gel after the initial cell culture.
The change in the refractive index of the silicone distorts things a little, so DIC will be possible but not perfect. Also, DIC is only possible on the glass bottom imaging plates, not the 6-well plastic plates.
1.41
The two main issues are:
1. Ineffecient coating of the matrix proteins due to low pH of the coating solution.
2. Insufficient wash of leftover matrix after the coating procedure.
3. After coating, plates needed to be allowed to incubate longer for improved ECM attachment.
We have mostly tested CytoSoft with ECM proteins such as collagen or fibronectin, but a few publications have recently come out using Poly-D-lysine and Poly-L-Lysine instead (while following the rest of the protocol as is).
The surface is decorated with anhydride functional groups.
Plasma use is not recommended. Plasma will induce formation of a hard crust on the surface and will change the mechanical properties of the CytoSoft® products.
Do not freeze the CytoSoft® products.
When frozen, there is a good chance that the silicone surface gets hydrolyzed and absorbs moisture, which would inactivate the binding sites and make the product not-usable.
If you froze the product and it is still frozen, warm the CytoSoft® up at 60C with the bag vented. That will minimize the chance of them absorbing moisture – but there is still a chance that they will no longer be functional.
Using gelatin for coating CytoSoft is often problematic because gelatin often has low molecular weight impurities that block binding sites on the activated surface of the silicone. We recommend using highly purified ECM's instead.
Fibroblast cells secrete their own ECM. The Silicone surface is not efficient for passive absorption of ECM and this is why we use covalent bonding to attach the ECM molecules to its surface for the initial coating.
Fibroblasts use the attached ECM molecules (from the initial coating) to attach and spread on the plate. Fibroblasts are very good at laying down new ECM and after prolonged culture they build a thick layer of ECM on the top of silicone surface.
The problem is that the cell-secreted ECM is not sticking very well to the silicone gel surface and is easy to be pulled by cellular forces. One way to prevent this from happening is to put some sort of 3D hydrogel on top of the cells after they attach so that the hydrogel absorbs or incorporates some of that secreted ECM. A possible downside would be that now the cells are attached to one stiffness (the silicone) but then surrounded by another (the hydrogel).
Click the links below to download the exact plate dimensions and specifications:
No. The humidity in the incubator will deactivate the binding sites on the silicone surface.
The silicone gel will absorb some of this dye. The absorption will be minimal when using the imaging plates.
For the most part, the dye will work just fine. To compensate for absorption, a higher concentration of dye than you would use on plastic may need to be used.
Lipid stains work on Imaging Plates but not on the plastic plates (ie. 6-well). The background stain will be very heavy for the plastic plates because too much dye binds to the thicker PDMS coating. The ~20 micron thickness PDMS on the imaging plates seems to not bind as much dye, allowing you to use the stains.
Yes - you can add Trizol directly to the plates.
The glass bottom for the Imaging plates is #1.5 cover glass thickness, and the layer of silicone coating the glass is thin enough to allow for high resolution imaging. Add oil to objective lens and place the Imaging plate close to the objective, per standard procedure.
Most standard assays performed on cultureware can be performed on CytoSoft plates. This publication may be a good reference:
Product Cell Assay
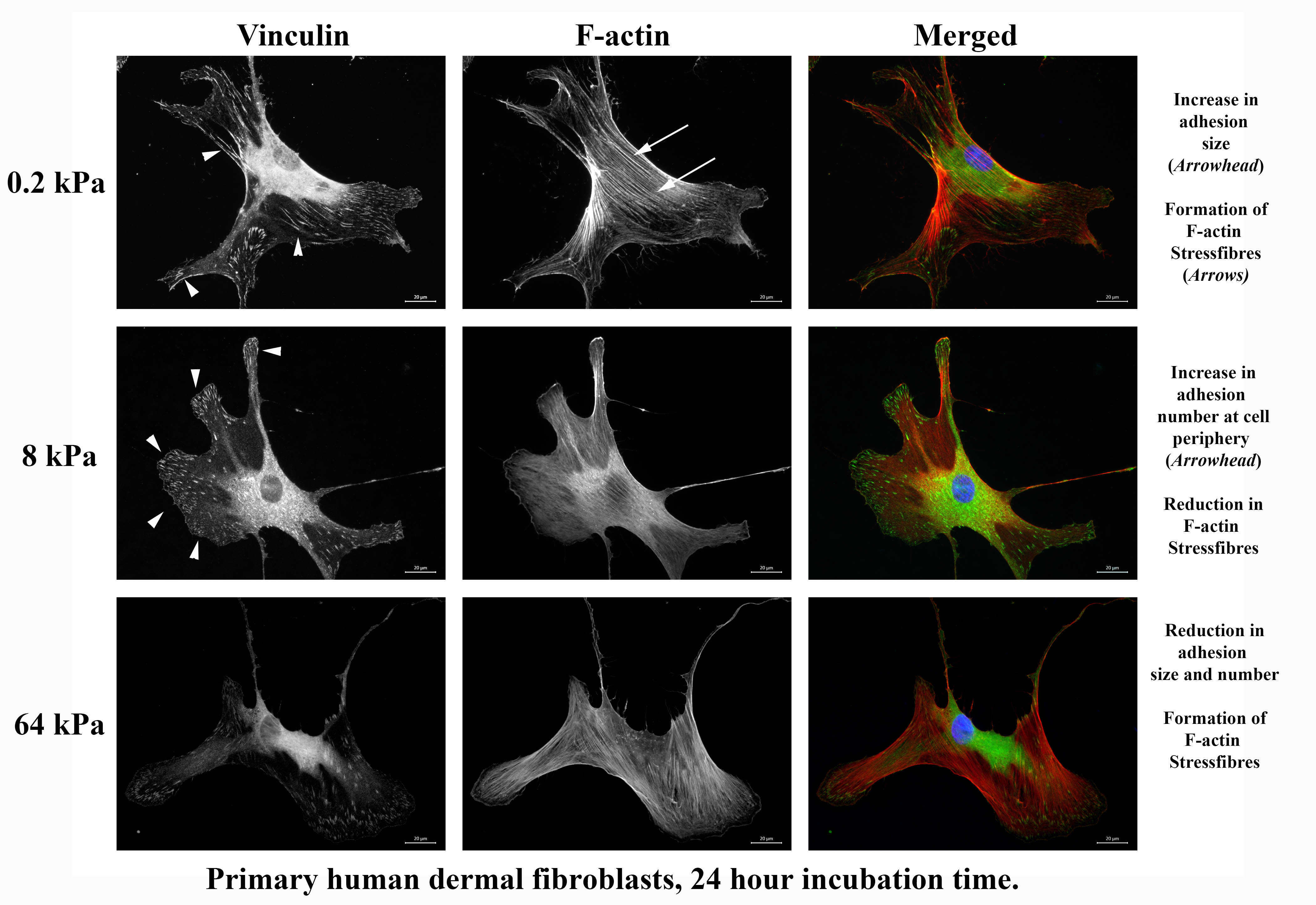
CytoSoft™ plates can also be used to show how fibroblasts are able to discriminate between the underlying stiffness. This is manifested in both adhesion size and stress fibers, as seen below. It appears that the cells on the 8 kPa stiffness have reduced intracellular tension and increased adhesion.
Product Applications
View our CytoSoft eBrochure Here
| Mesenchymal Stem Cells (1) | MSCs on soft substrates (1 kPa) had a higher expression of chondrogenic marker collagen-II and adipogenic marker lipoprotein lipase, less spreading, fewer stress fibers and lower proliferation rates than on hard substrates (15 kPa). |
| Fibroblasts (2) | PDGF-induced chemotaxis and migration of pulmonary fibroblasts precultured on stiff substrates (25 kPa) were significantly higher than those of cells precultured on soft gels (2 kPa). |
| Endothelial Cells (3) | Endothelial cell contraction-mediated neutrophil transmigration using an in vitro model of the vascular endothelium. Neutrophil transmigration increased with increasing substrate stiffness below the endothelium. |
| Neural Stem Cells (4) | NSCs proliferated on substrates below 10 kPa and exhibited maximal proliferation on 3.5 kPa surfaces. Neuronal differentiation was favored on the softest surfaces with EY < 1 kPa. Oligodendrocyte differentiation was favored on stiffer scaffolds >7 kPa. Astrocyte differentiation was only observed on <1 and 3.5 kPa surfaces and represented less than 2% of the total cell population. |
| Tumor Cells (5) | CCN1/CYR61 is highly regulated by stiffness in endothelial cells. Stiffness‐induced CCN1 activates β‐catenin nuclear translocation and signaling and that this contributes to upregulate N‐cadherin levels on the surface of the endothelium, increasing cancer cell metastasis. |
| Cancer Stem Cells (6) | Fibrotic rigidities found in pancreatic cancer tissues promote elements of EMT, including increases in vimentin expression, decreases in E-cadherin expression, nuclear localization of β-catenin, YAP and TAZ, changes in cell shape towards a mesenchymal phenotype and induction of chemoresistance. |
| Mammary Epithelium (7) | ECM stiffness alone induces malignant phenotypes but that the effect is completely abrogated when accompanied by an increase in basement-membrane ligands. |
| Hepatic Stellate Cells (8) | Higher matrix stiffness leads to transdifferentiation of hepatic stellate cells into fibrogenic, proliferative, and contractile myofibroblasts. |
| Cardiomyocytes (9) | Inverse correlation between the material stiffness and the amplitude of contraction of the cardiac tissue constructs by changing the modulus of the matrix using different compositions of PEG and fibrinogen. |
| Smooth Muscle Cells (10) | Elastic modulus between 448 and 5804 Pa regulated SMC cytoskeletal assembly in 3D, with cells in stiff matrices having a slightly higher degree of F-actin bundling after prolonged culture. |
- Song Li, et al. The Effect of Matrix Stiffness on the Differentiation of Mesenchymal Stem Cells in Response to TGF-β. Biomaterials. 2011 Jun; 32(16): 3921–3930.
- Hasegawa Y, et al. Matrix stiffness regulates migration of human lung fibroblasts. Physiol Rep. 2017 May;5(9).
- Helim Aranda-Espinoza, et al. Endothelial cell substrate stiffness influences neutrophil transmigration via myosin light chain kinase-dependent cell contraction. Blood. 2011 Aug 11; 118(6): 1632–1640.
- Molly S. Shoichet, et al. The effect of substrate stiffness on adult neural stem cell behavior. Volume 30, Issue 36, December 2009, Pages 6867-6878.
- Sara Zanivan, et al. Tumor matrix stiffness promotes metastatic cancer cell interaction with the endothelium. EMBO J. 2017 Aug 15; 36(16): 2373–2389.
- Río Hernández, et al. Matrix stiffness induces epithelial–mesenchymal transition and promotes chemoresistance in pancreatic cancer cells. Oncogenesis volume 6, page e352 (2017).
- David J. Mooney, et al. Extracellular matrix stiffness and composition jointly regulate the induction of malignant phenotypes in mammary epithelium. Nature Materials volume 13, pages 970–978 (2014).
- Wells RG, et al. The role of matrix stiffness in hepatic stellate cell activation and liver fibrosis. J Clin Gastroenterol. 2005 Apr;39(4 Suppl 2):S158-61.
- DrorSeliktar, et al. Matrix stiffness affects spontaneous contraction of cardiomyocytes cultured within a PEGylated fibrinogen biomaterial. Acta Biomaterialia. Volume 3, Issue 1, January 2007, Pages 33-41.
- Andrew J.Putnam, et al. The effects of matrix stiffness and RhoA on the phenotypic plasticity of smooth muscle cells in a 3-D biosynthetic hydrogel system. Biomaterials, volume 29, Issue 17, June 2008, Pages 2597-2607.
Product Certificate of Analysis
No result for .
Product Videos
Product Disclaimer
This product is for R&D use only and is not intended for human or other uses. Please consult the Material Safety Data Sheet for information regarding hazards and safe handling practices.