-
Collagen
-
Type I - Atelocollagen
- PureCol® Solution, 3 mg/ml (bovine) #5005
- Nutragen® Solution, 6 mg/ml (bovine) #5010
- FibriCol® Solution, 10 mg/ml (bovine) #5133
- PureCol® EZ Gel, Solution, 5 mg/ml (bovine) #5074
- PureCol® Lyophilized, 15 mg (bovine) #5006
- VitroCol® Solution, 3 mg/ml (human) #5007
- VitroCol® Lyophilized, 15 mg (human) #5008
-
Type I - Telocollagen
- TeloCol®-3 Solution, 3 mg/ml (bovine) #5026
- TeloCol®-6 Solution, 6 mg/ml (bovine) #5225
- TeloCol®-10 Solution, 10 mg/ml (bovine) #5226
- RatCol™ for 2D and 3D, Solution, 4 mg/ml (rat) #5153
- RatCol™ High Concentration, Solution, 10 mg/ml (rat)
- RatCol™ lyophilized, 100 mg (rat)
- RatCol™ for Coatings, Solution, 4 mg/ml (rat) #5056
- Type I - Insoluble Collagen
- Type I - Bioinks
- Type II Collagen
- Type III Collagen
- Type IV Collagen
- Collagen Standard
-
PureCol® Collagen Coated Plates
- Collagen Coated T-25 Flasks #5029
- Collagen Coated 6-well Plates #5073
- Collagen Coated 12-well Plates #5439
- Collagen Coated 24-well Plates #5440
- Collagen Coated 48-well Plates #5181
- Collagen Coated 96-well Plates #5072
- Collagen Coated 384-well Plates #5380-5EA
- Collagen Coated 100 x 20 mm Dishes #5028
- MatTek Glass-Bottom Dishes
- MatTek Multi-Well Plates
- Collagen Scaffolds
- Collagen Hybridizing Peptides
-
Type I - Atelocollagen
- Tunable Stiffness
- CytoSoft™ Rigidity Plates
-
Bioprinting
- Support Slurry for FRESH Bioprinting
-
Bioinks for Extrusion Bioprinting
- Lifeink® 200 Collagen Bioink (35 mg/ml) #5278
- Lifeink® 220 Collagen Bioink (70 mg/ml) #5343
- Lifeink® 240 Acidic Collagen Bioink (35 mg/ml) #5267
- Lifeink® 260 Acidic Collagen Bioink (70 mg/ml) #5358
- GelMA Bioink
- GelMA A Bioink
- GelMA C Bioink
- Pluronic F-127 40% Sterile Solution
- GelMA 20% Sterile Solution
- Alginate 5% Sterile Solution
- Photoinitiators
- Bioinks for BIONOVA X
- Bioinks for Lumen X
- DLP Printing Consumables
-
Create Your Own Bioinks
- PhotoCol® Methacrylated Collagen
- PhotoGel® Methacrylated Gelatin 95% DS
- PhotoGel® Methacrylated Gelatin 50% DS
- PhotoHA®-Stiff Methacrylated Hyaluronic Acid
- PhotoHA®-Soft Methacrylated Hyaluronic Acid
- PhotoAlginate® Methacrylated Alginate
- PhotoDextran® Methacrylated Dextran
- PEGDA (Various Molecular Weights)
- Silk Fibroin, Solution
- PhotoSericin® Methacrylated Sericin
- Bioprinters
-
3D Hydrogels
- Thermoreversible Hydrogel
- Silk Fibroin
-
Type I Collagen for 3D Hydrogels
- PureCol® Solution, 3 mg/ml (bovine) #5005
- Nutragen® Solution, 6 mg/ml (bovine) #5010
- FibriCol® Solution, 10 mg/ml (bovine) #5133
- PureCol® EZ Gel, Solution, 5 mg/ml (bovine) #5074
- VitroCol® Solution, 3 mg/ml (human) #5007
- TeloCol®-3 Solution, 3 mg/ml (bovine) #5026
- TeloCol®-6 Solution, 6 mg/ml (bovine) #5225
- TeloCol®-10 Solution, 10 mg/ml (bovine) #5226
- RatCol® for 3D gels, Solution, 4 mg/ml (rat) #5153
- HyStem® Thiolated Hyaluronic Acid
- Methacrylated Collagen
- Methacrylated Gelatin
- Methacrylated Hyaluronic Acid
- Diacrylates
- Collagen Sponges
- Methacrylated Polysaccharides
- Spheroids and Organoids
- Extracellular Matrices
- HyStem / Hyaluronic Acid
-
Adhesion Peptides / Proteins
-
Recombinant Adhesion Proteins
- CD2, 0.5 mg/ml #5086
- CDH3, 0.5 mg/ml #5124
- CDH13, 0.5 mg/ml #5125
- CD14, 0.5 mg/ml #5089
- CDH18, 0.5 mg/ml #5090
- CD40, 0.5 mg/ml #5093
- CD86, 0.5 mg/ml #5096
- CD164, 0.5 mg/ml #5100
- CD270, 0.5 mg/ml #5127
- CD274, 0.5 mg/ml #5126
- CD276, 0.5 mg/ml #5123
- E-Cadherin (CD324), 0.5 mg/ml #5085
- ICAM2, 0.5 mg/ml #5107
- Adhesion Peptides
- Collagen Hybridizing Peptides
-
Recombinant Adhesion Proteins
- Reagents
- Assays
Ruthenium
Visible Light Photoinitiator (400-450nm)
Catalog #5248
Ruthenium
Visible Light Photoinitiator (400-450nm)
Catalog #5248
Ruthenium is a water soluble photoinitiator that utilizes visible light (400-450nm) to covalently crosslink free tyrosine and acryl groups. Ruthenium has been tested on collagen type I, gelatin, silk fibroin, methacrylated hyaluronic acid, methacrylated gelatin, methacrylated collagen type I and PEGDA.
Product Description
The Ruthenium visible light photocrosslinking kit is ideal for tissue engineering, cell culture, and bioprinting, where tuning the mechanical properties of the substrate is required. The kit provides enough photoinitiator for >200 mL of bioinks/hydrogels (following recommended concentrations).
- Ruthenium is a photoinitiator that utilizes visible light photocrosslinking (400-450nm) to covalently crosslink free tyrosine and acryl groups.
- Ruthenium photoinitiator has been tested on collagen type I, gelatin, silk fibroin, methacrylated hyaluronic acid, methacrylated gelatin, methacrylated collagen type I and PEGDA.
- Ruthenium is water soluble and yields better cell cytocompatibility, and crosslinking efficiency.
- Increasing Visible light intensity from 3-100 mW/cm2 using Ruthenium did not significantly decrease cell viability from 90%.
- Increasing Ruthenium concentration by 10 times (10x) did not decrease cell viability from 90%.
- Ruthenium is red/yellow/orange in color and will change the color of your solutions, hydrogels, or printed constructs.
This Ruthenium photocrosslinking kit is considered non-sterile. Adding antibiotics to your cell culture system, or sterile filtering is recommended. To sterile filter, resuspend the entire volume of Ruthenium and Sodium Persulfate (separately) and filter through small 0.2 micron button filters (separately). Use the sterile photoinitiator within 2 weeks.
The Ruthenium visible light photocrosslinking kit is composed of two components, as found in Table 1.
Table 1:
| Item | Catalog Number | Package Size |
| Ruthenium Photoinitiator | #5246 | 200 mg |
| Sodium Persulfate Photoinitiator | #5247 | 1 gram |
Storage/Stability:
The product ships ambient. Store the kit at room temperature. Weigh out the required amount of powder to solubilize. Once solubilized, use the Ruthenium and Sodium Persulfate within 2 weeks.
Dry powder (non-solubilized) is stable for >1 year at room temperature.
Directions for Use
Download the full PDF version or continue reading below:
1. Calculate desired volume of hydrogel or bioink (ECM + cells).
2. Multiply desired volume by 0.02. This is how much Ruthenium and Sodium Persulfate (each) you will be adding to your pre-hydrogel solution.
3. Solubilize required Ruthenium in water or 1X PBS at a concentration of 37.4 mg/mL.
4. Solubilize Sodium Persulfate in water or 1X PBS at a concentration of 119 mg/mL.
5. Add the calculated volume (step 2) of Ruthenium to your pre-hydrogel solution and thoroughly mix.
6. Add the calculated volume (step 2) of Sodium Persulfate to your pre-hyrogel solution and thoroughly mix.
Notes:
Do not mix the Ruthenium and Sodium Persulfate together prior to adding to the pre-hydrogel. You will get a rapid redox reaction and they will precipitate instantaneously.
7. Add in cells, if desired.
8. Photocrosslink at 400-450nm wavelength. Initial recommendation is 50 mW/cm2 for >3 minutes to maintain shape/hydrogel fidelity. You may tune photoinitiator concentration, light intensity and Photocrosslinking time to customize final hydrogel stiffness.
Additional Notes:
If using neutralized type I collagen, you can allow the collagen to polymerize at 37C to form a hydrogel, and then photocrosslink to further crosslink and modulate the gel stiffness.
How to use Ruthenium with Lifeink® 200 and 3D Bioprinting:
1. Print Lifeink® 200 according to the recommended protocols, into the FRESH support slurry.
2. After printing, incubate the print to melt the FRESH gelatin support slurry.
3. After ~30 minutes, replace the melted gelatin with warm cell culture media.
4. For example, pipette out 2 mL of melted gelatin, and then add in 2 mL of media. Repeat until the gelatin is removed.
5. Prepare stock solutions of Ruthenium and Sodium Persulfate (found in the above protocol).
6. Multiply total volume of media that your Lifeink® 200 structure is floating in. Multiply by 2%, and add that amount of Ruthenium and Sodium Persulfate to the cell culture media (directly in the same dish that your structure is in).
7. Photocrosslink with visible light (400-450 nm) until desired crosslinking is achieved.
8. Gently replace the media with fresh media, as done in step 3.
Product Q & A
The full abosrbance spectrum can be found here: https://iopscience.iop.org/1758-5090/10/3/034101/media/BFaac00c_suppdata.pdf
Ruthenium: 748.62
Sodium Persulfate: 238.10
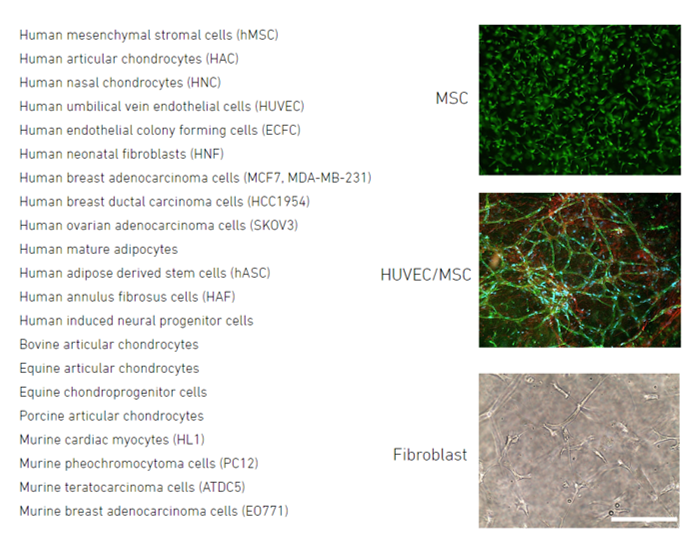
Product Cell Assay
Cells tested within 3D hydrogels crosslinked with Ruthenium and visible light photocrosslinking:
Product References
References for Ruthenium:
1. Parrish, J., Lim, K. S., Baer, K., Hooper, G. J., & Woodfield, T. B. F. (2018). A 96-well microplate bioreactor platform supporting individual dual perfusion and high-throughput assessment of simple or biofabricated 3D tissue models. Lab on a Chip. Advance online publication. doi: 10.1039/c8lc00485d
2. Parker, J. D., Lim, K. S., Kieser, D. C., Woodfield, T. B. F., & Hooper, G. J. (2018). Is tranexamic acid toxic to articular cartilage when administered topically? What is the safe dose? Bone & Joint Journal, 100-B(3), 404-412. doi: 10.1302/0301-620X.100B3.BJJ-2017-1135.R1
3. Lim, K. S., Levato, R., Costa, P. F., Castilho, M. D., Alcala-Orozco, C. R., van Dorenmalen, K. M. A., … Hooper, G. J., … Woodfield, T. B. F. (2018). Bio-resin for high resolution lithography-based biofabrication of complex cell laden constructs. Biofabrication, 10, 034101. doi: 10.1088/1758-5090/aac00c
4. Bertlein, S., Brown, G., Lim, K. S., Jungst, T., Boeck, T., Blunk, T., … Hooper, G. J., Woodfield, T. B. F., & Groll, J. (2017). Thiol-ene clickable gelatin: A platform bioink for multiple 3D biofabrication technologies. Advanced Materials, 29(44), 1703404. doi: 10.1002/adma.201703404
5. Mekhileri, N. V., Lim, K. S., Brown, G. C. J., Mutreja, I., Schon, B. S., Hooper, G. J., & Woodfield, T. B. F. (2017). Automated 3D bioassembly of micro-tissues for biofabrication of hybrid tissue engineered constructs. Biofabrication. Advance online publication. doi: 10.1088/1758-5090/aa9ef1
6. Lim, Khoon S., et al. "New visible-light photoinitiating system for improved print fidelity in gelatin-based bioinks." ACS Biomaterials Science & Engineering 2.10 (2016): 1752-1762.
7. Lim, K. S., Ramaswamy, Y., Roberts, J. J., Alves, M.-H., Poole-Warren, L. A., & Martens, P. J. (2015). Promoting cell survival and proliferation in degradable poly(vinyl alcohol)-tyramine hydrogels. Macromolecular Bioscience, 15(10), 1423-1432. doi: 10.1002/mabi.201500121
8. Lim, K. S., Alves, M. H., Poole-Warren, L. A., & Martens, P. J. (2013). Covalent incorporation of non-chemically modified gelatin into degradable PVA-tyramine hydrogels. Biomaterials,34(29), 7097-7105. doi: 10.1016/j.biomaterials.2013.06.005
9. Green, R. A., Lim, K. S., Henderson, W. C., Hassarati, R. T., Martens, P. J., Lovell, N. H., & Poole-Warren, L. A. (2013). Living electrodes: Tissue engineering in the neural interface. Proceedings of the 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS). (pp. 6957-6960). IEEE. doi: 10.1109/EMBC.2013.6611158
Product Certificate of Analysis
No result for .
Product Disclaimer
This product is for R&D use only and is not intended for human or other uses. Please consult the Material Safety Data Sheet for information regarding hazards and safe handling practices.