-
Collagen
-
Type I - Atelocollagen
- PureCol® Solution, 3 mg/ml (bovine) #5005
- Nutragen® Solution, 6 mg/ml (bovine) #5010
- FibriCol® Solution, 10 mg/ml (bovine) #5133
- PureCol® EZ Gel, Solution, 5 mg/ml (bovine) #5074
- PureCol® Lyophilized, 15 mg (bovine) #5006
- VitroCol® Solution, 3 mg/ml (human) #5007
- VitroCol® Lyophilized, 15 mg (human) #5008
-
Type I - Telocollagen
- TeloCol®-3 Solution, 3 mg/ml (bovine) #5026
- TeloCol®-6 Solution, 6 mg/ml (bovine) #5225
- TeloCol®-10 Solution, 10 mg/ml (bovine) #5226
- RatCol® for 2D and 3D, Solution, 4 mg/ml (rat) #5153
- RatCol® High Concentration, Solution, 10 mg/ml (rat)
- RatCol® lyophilized, 100 mg (rat)
- RatCol® for Coatings, Solution, 4 mg/ml (rat) #5056
- Type I - Insoluble Collagen
- Type I - Bioinks
- Type II Collagen
- Type III Collagen
- Type IV Collagen
- Collagen Standard
-
PureCol® Collagen Coated Plates
- Custom-Coated Cultureware and Plates
- Collagen Coated T-25 Flasks #5029
- Collagen Coated 6-well Plates #5073
- Collagen Coated 12-well Plates #5439
- Collagen Coated 24-well Plates #5440
- Collagen Coated 48-well Plates #5181
- Collagen Coated 96-well Plates #5072
- Collagen Coated 384-well Plates #5380-5EA
- Collagen Coated 100 x 20 mm Dishes #5028
- MatTek Glass-Bottom Dishes
- MatTek Multi-Well Plates
- Collagen Scaffolds
- Collagen Hybridizing Peptides
-
Type I - Atelocollagen
- Tunable Stiffness
- CytoSoft® Rigidity Plates
-
Bioprinting
- Support Slurry for FRESH Bioprinting
-
Bioinks for Extrusion Bioprinting
- Lifeink® 200 Collagen Bioink (35 mg/ml) #5278
- Lifeink® 220 Collagen Bioink (70 mg/ml) #5343
- Lifeink® 240 Acidic Collagen Bioink (35 mg/ml) #5267
- Lifeink® 260 Acidic Collagen Bioink (70 mg/ml) #5358
- GelMA Bioink
- GelMA A Bioink
- GelMA C Bioink
- Pluronic F-127 40% Sterile Solution
- GelMA 20% Sterile Solution
- Alginate 5% Sterile Solution
- Photoinitiators
- Bioinks for BIONOVA X
- Bioinks for Lumen X
- DLP Printing Consumables
-
Create Your Own Bioinks
- PhotoCol® Methacrylated Collagen
- PhotoGel® Methacrylated Gelatin 95% DS
- PhotoGel® Methacrylated Gelatin 50% DS
- PhotoHA®-Stiff Methacrylated Hyaluronic Acid
- PhotoHA®-Soft Methacrylated Hyaluronic Acid
- PhotoAlginate® Methacrylated Alginate
- PhotoDextran® Methacrylated Dextran
- PhotoChitosan® Methacrylated Chitosan
- PEGDA (Various Molecular Weights)
- Silk Fibroin, Solution
- PhotoSericin® Methacrylated Sericin
- Bioprinters
-
3D Hydrogels
- Thermoreversible Hydrogel
- Silk Fibroin
-
Type I Collagen for 3D Hydrogels
- PureCol® Solution, 3 mg/ml (bovine) #5005
- Nutragen® Solution, 6 mg/ml (bovine) #5010
- FibriCol® Solution, 10 mg/ml (bovine) #5133
- PureCol® EZ Gel, Solution, 5 mg/ml (bovine) #5074
- VitroCol® Solution, 3 mg/ml (human) #5007
- TeloCol®-3 Solution, 3 mg/ml (bovine) #5026
- TeloCol®-6 Solution, 6 mg/ml (bovine) #5225
- TeloCol®-10 Solution, 10 mg/ml (bovine) #5226
- RatCol® for 3D gels, Solution, 4 mg/ml (rat) #5153
- HyStem® Thiolated Hyaluronic Acid
- Methacrylated Collagen
- Methacrylated Gelatin
- Methacrylated Hyaluronic Acid
- Diacrylates
- Collagen Sponges
- Methacrylated Polysaccharides
- Spheroids and Organoids
- Extracellular Matrices
- HyStem / Hyaluronic Acid
-
Adhesion Peptides / Proteins
-
Recombinant Adhesion Proteins
- CD2, 0.5 mg/ml #5086
- CDH3, 0.5 mg/ml #5124
- CDH13, 0.5 mg/ml #5125
- CD14, 0.5 mg/ml #5089
- CDH18, 0.5 mg/ml #5090
- CD40, 0.5 mg/ml #5093
- CD86, 0.5 mg/ml #5096
- CD164, 0.5 mg/ml #5100
- CD270, 0.5 mg/ml #5127
- CD274, 0.5 mg/ml #5126
- CD276, 0.5 mg/ml #5123
- E-Cadherin (CD324), 0.5 mg/ml #5085
- ICAM2, 0.5 mg/ml #5107
- Adhesion Peptides
- Collagen Hybridizing Peptides
-
Recombinant Adhesion Proteins
- Reagents
- Assays
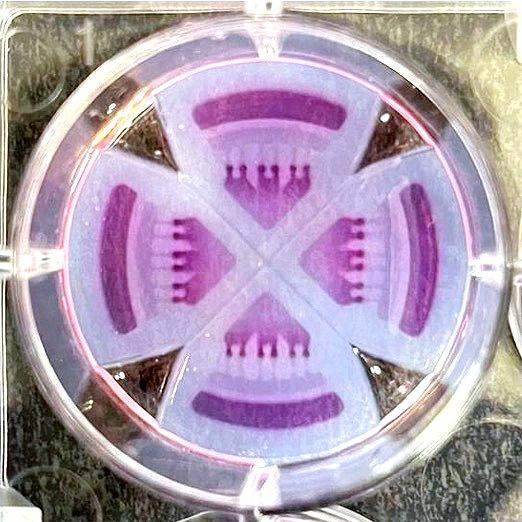
3-in-1 Plate
Spheroid, Organoid and Testing
Catalog #5434

3-in-1 Plate
Spheroid, Organoid and Testing
Catalog #5434
The 3-in-1 Plate is a hydrogel insert that fits within each well of a six-well plate. The insert combines spheroid/organoid formation, extracellular matrix integration and drug testing all in one platform.
Product Description
Background:
The 3-in-1 Plate is a hydrogel insert that fits within each well of a six-well plate. The insert combines spheroid/organoid formation, extracellular matrix integration and drug testing all in one platform.
The hydrogel comes pre-inserted within each well of a standard six-well plate. Each insert contains 4 quadrants, allowing for the formation of 6 spheroids/organoids per quadrant. The six well plate can be used for 144 spheroids/organoids.
Each quadrant is separated from one another, so researchers may run 4 different experimental conditions on one insert.
The hydrogel is a non-cell-adhesive hydrogel.
The 3-in-1 plate benefits:
- Culture spheroids or organoids (mono/co-cultured), embed them in an extracellular matrix, test drugs & perform downstream analysis. All without ever having to remove them from the platform.
- Low spheroid/organoid size variance increases experimental consistency.
- Only one spheroid/organoid per microwell. Eliminates any possibility of inter-model crosstalk.
- Disturbance free media changes. No risk of aspirating or disrupting the spheroids while changing the media.
- Low cell requirements reduces the need to passage cells beforehand.
- Compatible with in situ immunostaining and imaging.
- In situ IHC analysis. Researchers can perform all steps of IHC analysis – from dehydration to wax embedding and slicing - without ever having to remove the spheroids from the platform.
Additional specifications:
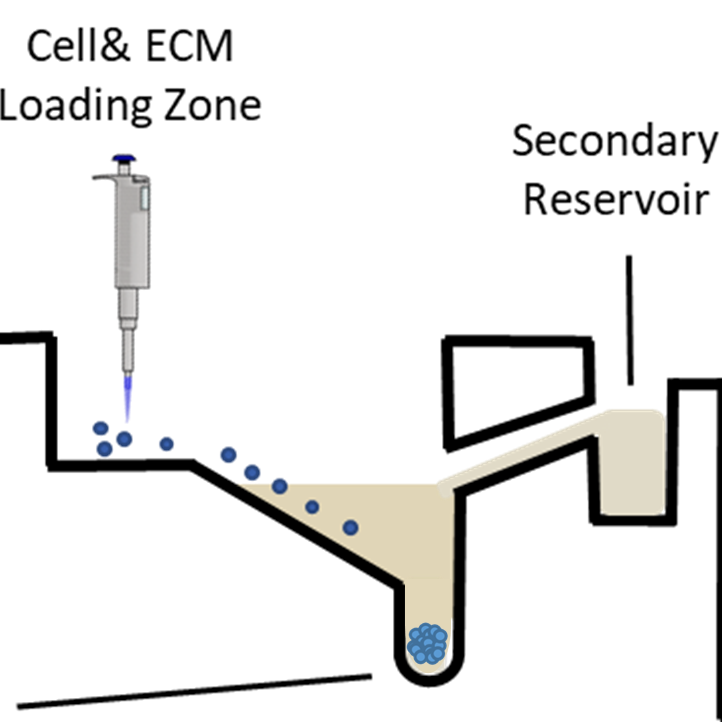
Microwell diameter: 800 um
Microwell volume: 13 uL
Volume of media reservoir: 300 uL
Secondary resevoir volume: 20 uL
Directions for Use
Directions for Use:
Additional Suggestions Below:
Spheroid Formation
The geometry and material of the microwells in the 3-in-1 Plate facilitate the formation of compact and uniform spheroids/organoids. However, not all cells will readily form tight spheroids. There are some tips and techniques that can aid in spheroid formation. Specifically, optimizing the spheroid seeding density, media formulation, culture period, or adding an overlay of extracellular matrix (ECM) hydrogel can aid in tighter spheroid formation.
Spheroid Size
Spheroid size is determined by the microwell size, cell type, seeding density, and culture time. Optimizing the initial seeding density will play a large role in the ability of the spheroids to initially form, as well as determine how long the spheroids can be kept in culture. Larger spheroids can begin to form hypoxic cores, which may or may not be desirable based on your experiment.
Media Formulation
Media formulation may affect spheroid formation. This should be tested with each cell type.
Removing The Insert
Press a lab spatula into the gap between the insert and the plastic of the well plate. Once at the bottom of the six-well plate, flex your lab spoon towards the center, this motion should pop the insert out of the well. Securely support the insert with the lab spatula and remove from the six-well plate.
Extracellular Matrices Embedding
Some applications require the addition of an extracellular matrix (ECM) hydrogel. A primary ECM can be added to the Cell & ECM Loading Zone. For co-cultures (such as fibroblasts and tumor cells) or tri-cultures, a primary or secondary ECM overlay can be added to the Secondary Reservoir. The ECM concentration should be optimized for the specific application. Organoid cultures typically call for undiluted Matrigel matrix in the concentration range of 8 to 10 mg/mL, while other applications call for a more dilute concentration. When adding an ECM to the 3-in-1 Plate, users must ensure that the ECM is not excessively viscous – it must be able to flow down the microchannels leading to the microwells. The ECM can be added during spheroid formation, or as an overlay to an already formed spheroid. Media should be aspirated before adding an ECM. This can be done by aspirating media from the Media Reservoir and Cell & ECM Loading Zone. Then, waiting 3-5 minutes to ensure that any remaining media is absorbed by the hydrogel of the 3-in-1 Plate.
Spheroid Handling
Media/Buffer Exchanges
The 3-in-1 Plate eliminates the possibility of disrupting or aspirating the spheroids during media changes. To change the media, pipette from the Media Reservoir. Any additional media left in the microwells can be pipetted out from the Cell & ECM Loading Zone. Complete aspiration of the media is not required when exchanging media/buffer.
Spheroid Transfer
Spheroids can be cultured, embedded in an extracellular matrix, imaged, stained and used for drug screening without having to remove them from the 3-in-1 Plate. However, if necessary, spheroids can be harvested by gently flushing out the spheroids with 500 μL of culture media.
Centrifugation
Centrifugation is not required for spheroid formation or centering within the microwells.
Single Cell Recovery
For assays requiring single cell suspensions, spheroids can be dissociated in the microwells by incubating with reagents such as Accutase®, 5 mM EDTA, 1X Trypsin/EDTA, or 1X, 5X, or 10X TrypLE TM, or a combination of collagenase and a certain amount of 1X Trypsin. Users can add the appropriate dissociation reagents to the Media Reservoir and place in the incubator. The cell suspension can then be aspirated from the microchannels. Using a brightfield microscope would help ensure that all the dissociated cells are aspirated.
Product Q & A
Each insert has 24 spheroids (4 different quadrants with 6 spheroids/organoids per quadrant). Each quadrant is separated from one another, so you can run 4 different experimental conditions in the one insert.
There will only ever be one spheroid or organoid per microwell.
The hydrogel used to form the 3-in-1 Plate is non-cell adherent. Occasionally cells will become stuck in the channels that lead to the microwells. If this occurs, gently rinse any additional cells stuck in the channels by adding 250-300 μL of pre-warmed medium to the Cell & ECM Loading Zone. In most instances, cells that become stuck in the channels will naturally slide down the channels during the incubation period over the course of a few hours.
There may be a small amount of media left in the microwells after pipetting from the Media Reservoir. If this occurs, simply aspirate the remaining media from the Cell & ECM Loading Zone.
Yes. The plate can be placed on ice. We do not recommend placing it on ice for more than 20 minutes at a time. Freezing our platform is not recommended as it can distort the delicate microwell structure and reduce spheroid consistency.
The size of the spheroids/organoids is determined by a variety of factors: the quantity of cells that are seeded, the type of cells, the size of the microwells and the duration of culture.
Adding an ECM hydrogel is not always necessary, depending on the type of model you wish to create. Spheroids can be formed without a hydrogel. However, culturing primary cells to form organoids typically requires an ECM hydrogel.
The Secondary Reservoir has been optimized for certain co-culture and tri-culture experiments. For example, fibroblast co-culture with tumor cells. It is also ideal for experiments that require a secondary ECM, such as in many tri-culture experiments. For these types of experiments, a primary ECM should be added to the Cell & ECM Loading Zone, and a secondary ECM overlay should be added to the Secondary Reservoir.
The 3-in-1 Plate is compatible with all standard forms of microscopy: inverted, upright, bright field, fluorescent, confocal, etc.
Yes. In fact, an inverted microscope is ideal. For improved imaging the inserts can be removed from the well and placed on a coverslip.
No. Users have not reported any distortion/reflection for imaging.
Yes, as long as it doesn’t affect cell proliferation.
Yes. The 3-in-1 Plate is compatible with confocal microscopy and all staining can be done within the insert. For getting higher resolution images in confocal imaging, we recommend removing the insert from its well using a lab spatula and placing it on the coverslip. To remove the insert, press the lab spatula into the gap between the insert and the plastic of the well plate. Once at the bottom of the six-well plate, flex your lab spoon towards the center, this motion should pop the insert out of the well. Securely support the insert with the lab spatula and remove from the six-well plate.
The hydrogel type, cross-linking and concentration and time are all factors that can impact the matrix stiffness. What ECM hydrogel you choose for your experiment is ultimately up to you. The 3-in-1 Plate should be compatible with whatever you end up choosing. Ensure that the viscosity of the hydrogel is not excessively viscous when pipetting into the Cell & ECM Loading Zone.
All staining can be done on the plate. There is no need to remove the spheroids or even to digest the extracellular matrix.
No, the inserts are press fit into the well and are designed not to move even when media is added. The inserts are designed with tight tolerances to the exact dimensions of the well such that friction holds the inserts in place.
The wall design between the quadrants prevents media and added compounds from mixing from one to another quadrant. Cross-contamination may occur if the plate were bumped or too much media added, spilling media over the barrier into other quadrants.
By following the Cell & ECM Seeding Protocol, cells will evenly distribute across the microwells. For diluted cell suspension, for example, at < 50 k cells/50uL), it is recommended to keep the pipette tip close to the microwell section for even cell distribution.
The Apricell inserts provide a ULA material to grow spheroids. The design enables disturbance-free media changes, co-culture ability, and a flat bottom surface which helps generate images with increased clarity when compared to round bottom and hanging drop plates. A total of 144 spheroids may be produced in each plate, with six replicates per isolated quadrant. In addition, without transferring from the spheroids to other plates, extracellular matrices may be added directly to tumoroids and the insert may directly be sliced for immunohistochemistry analysis.
Product Cell Assay
Spheroid Assays
The transparent hydrogel of the 3-in-1 Plate makes it ideal for numerous fluorescent, and colorimetric assays that can be conducted directly in each insert. Users can add reagents to the Media Reservoir. Depending on the size of the spheroid it may become difficult for some assay reagents to penetrate fully. Therefore, optimization of each assay is recommended using appropriate positive and negative controls.
Homogenous Assays
There are several commercially available 3D-specific reagents that have been optimized for use with spheroids.
We recommend CellTiter-Glo® 3D cell viability assay (Promega Cat. No. G9683) or PrestoBlue™ Cell Viability assay (Invitrogen™) for enumerating total ATP content of spheroids.
Brightfield Imaging
The transparent hydrogel of the 3-in-1 Plate enables clear brightfield images and is compatible with both upright and inverted microscopy. We recommend using inverted microscopy due to the nature of our microwell design. Spheroids are much closer to the bottom of the plate, as such, inverted microscopy produces clearer images with reduced background particles compared to upright microscopy.
Fluorescent Imaging
Staining a 3D structure may require protocol optimization compared to the 2D equivalent. In general, the larger
and tighter the spheroid, the longer and more complex it will be for complete staining to occur. If cell permeabilization is required, reagent choice, and length of incubation time may need to be considered. We have had success using a variety of stains including primary and secondary conjugated antibodies with cells cultured in the 3-in-1 Plate. You can also pre-label cells prior to seeding in the 3-in-1 Plate, or use fluorescent protein expressing cells in order to ensure that all cells are labeled as needed for the application.
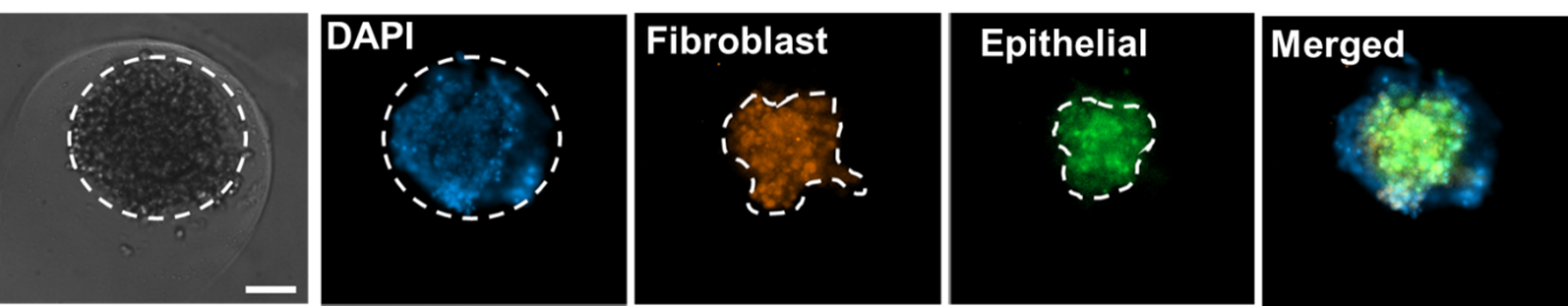
Fluorescent imaging of co-cultured tumor spheroids containing pancreatic/ fibroblast cells stained with fibronectin (red), E-Cadherin (green), and DAPI (Blue).
Immunohistochemistry Analysis
The 3-in-1 Plate is compatible with in situ IHC analysis. Because the 3-in-1 Plate is made from a soft hydrogel, users can perform all steps of IHC analysis – from dehydration to wax embedding and slicing - without having to remove the spheroids from the insert. Note: it is recommended that the dehydration step in ethanol is extended by 1.5 to 2 times the original duration to completely remove water from the insert hydrogel material. Refer to the 3-in-1 Plate IHC Analysis Protocol for specific instructions.
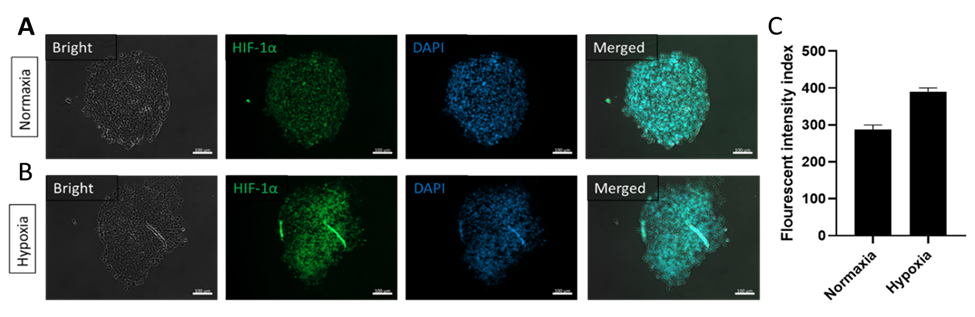
IHC analysis of the glioblastoma tumor slices in the 3-in-1 Plate under A) Normaxia conditions of tumor spheroids and B) Tumor spheroids placed in hypoxic conditions to simulate tumor conditions in the body. C) HIF-1α quantitates the expression of transcription factors under reduced O2 concentrations in mammalian cells.
Co-culture
Multiple cell types can be cultured together in a variety of ways to study cell-cell interactions, create
more in-vivo like models, or add structure to a cell line that does not easily form a tight spheroid. The cells can
be seeded at the same time or added at different time points depending on the application. Depending on the cell types used, media formulations may require optimization to provide sufficient nutrients and growth factors to specialized cell types included in the co-culture environment.
There are three ways to do a co-culture in the 3-in-1 Plate:
- By mixing the two cell types together as the initial single cell suspension and co-seeding that mixture.
- By first forming spheroids, then mixing secondary cells with an ECM hydrogel and applying that mixture to the spheroids.
- By mixing primary and secondary cells in an ECM hydrogel and seeding that mixture (ideal for organoid co-cultures).
Additionally, users may add a secondary extracellular matrix to the Secondary Reservoir. This is optimal for fibroblast co-cultures or tri-cultures that require a different matrix.
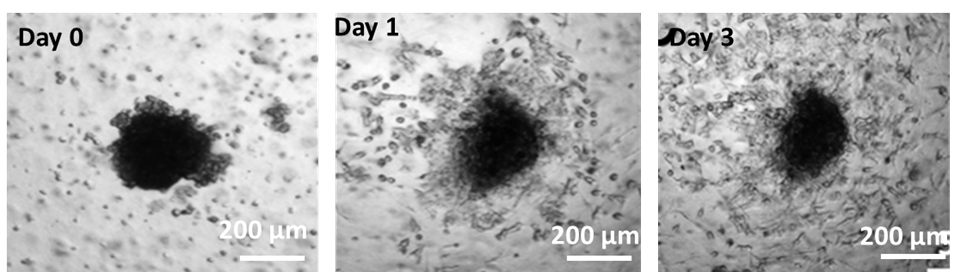
Multi-day imaging of SKOV-3 ovarian tumor spheroids embedded in a Collagen ECM containing normal human-derived fibroblasts (co-culture option 2).
Drug Screening
The 3-in-1 Plate is compatible with both small molecule and large molecule medium throughput drug screening. Simply mix your drug with media to the desired concentration and add it to the Media Reservoir.
Invasion Assay
The 3-in-1 Plate offers a flexible and high-throughput format for quantitating the degree of cell invasion/migration across microchannels in response to chemoattractant and/or inhibiting compounds. Chemoattractants, inhibitors, and/or migration regulating compounds can be added to ECM solutions when embedding spheroids or mixed with media to the desired working concentrations. The ECM / drug combination should be added to the Cell & ECM Loading Zone whereas the drug / media combination should be added to the Media Reservoir.
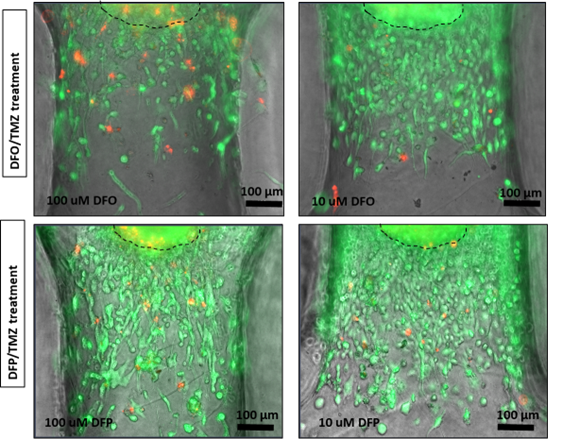
Images of Glioblastoma tumor spheroids invading within an ECM through the microchannels leading to each microwell of the 3-in-1 Plate. The effect of drug treatment on the invasion length of the tumor spheroids can be easily monitored under a brightfield and florescent microscope.
Product References
1.Chehri, B., Liu, K., Vaseghi, G., Seyfoori, A. & Akbari, M. In vitro glioblastoma model on a plate for localized drug release study from a 3D-printed drug-eluted hydrogel mesh. Cells 13, 363 (2024).
Product Certificate of Analysis
No result for .
Product Disclaimer
This product is for R&D use only and is not intended for human or other uses. Please consult the Material Safety Data Sheet for information regarding hazards and safe handling practices.